
���ۼ� | ����/kJ��mol-1 | ���ۼ� | ����/kJ��mol-1 |
H��H | 436 | O��H | 467 |
Cl��Cl | 243 | N��N | 945 |
C��H | 413 | H��Cl | 431 |
��1��H��H�ļ���Ϊʲô��Cl��Cl�ļ��ܴ�
��2����֪H2O��2
��3���Խ��͵���Ϊʲô���ڿ������ȶ����ڣ�
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� |
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��10�֣�Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ�ķ�Ӧ�ȣ�����ȡ��Ӧ��ʩ����ѧ��Ӧ�ķ�Ӧ��ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
��1�� ʵ���ã�5g�״��������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ����������״��ı�ȼ���Ȧ�H�� ��
��2���������������Ȼ�ѧ����ʽ��?��a b���������������������
H2(g)+ 1/2O2(g)��H2O(g)�� ��H1��a kJ��mol-1?
H2(g)+ 1/2O2(g)��H2O(l) ��H2��b kJ��mol-1?
��3����1mol��̬������ij�ֹ��ۼ���Ҫ���յ������м��ܡ��ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡��ڻ�ѧ��Ӧ�����У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ�������
|
��ѧ�� |
H��H |
N��H |
N��N |
|
����/kJ��mol��1 |
436 |
391 |
945 |
��֪��ӦN2(g)��3H2(g)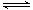 2NH3(g) ��H��a
kJ��mol��1���Ը��ݱ������м������ݹ���a
��ֵ��_______________(ע����+������)��
2NH3(g) ��H��a
kJ��mol��1���Ը��ݱ������м������ݹ���a
��ֵ��_______________(ע����+������)��
��4�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ�ķ�Ӧ�Ƚ������㡣����ˮú���ϳɶ����ѵ�������Ӧ���£�
�� 2H2(g) + CO(g)  CH3OH(g)����H ����90.8 kJ��mol��1
CH3OH(g)����H ����90.8 kJ��mol��1
�� 2CH3OH(g)
 CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
��
CO(g) + H2O(g)  CO2(g)
+ H2(g)����H����41.3 kJ��mol��1
CO2(g)
+ H2(g)����H����41.3 kJ��mol��1
�ܷ�Ӧ��3H2(g)
+ 3CO(g)  CH3OCH3(g) + CO2 (g)�Ħ�H�� ��
CH3OCH3(g) + CO2 (g)�Ħ�H�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�켪��ʡ��һ��ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ������
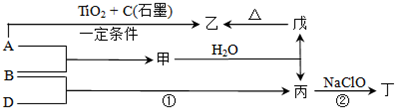
��A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵĺ˵������C��F��A��B��D��E��˳��������֪E��Fλ��ͬһ���壻C��D���ֱܷ���A��ԭ�Ӹ�����Ϊ1��1 ��2��1�γɻ����CB��һ��10e- ����̬���ӣ�E��M���������K���������2����
(1)д������Ԫ�ص����ƣ�B ��E ��F ��
(2)д��E��Ԫ�����ڱ��е�λ�õ� ���� �� �壻
(3)д��D2A2�ĵ���ʽ ��EB4
(4)�õ���ʽ��ʾFA2���γɹ��� ��
(5)�Ƚ�Ԫ��E��F�ķǽ�����ǿ����E�ķǽ����� ��F���ǿ���������������û�ѧ����ʽ֤���������� ��
(6)����A��B��C��D��ijЩԪ�ؿ��γɵļȺ����Ӽ��ֺ��Ǽ��Թ��ۼ��Ļ������� ���Ⱥ����Ӽ��ֺ����Թ��ۼ��Ļ������� ����д��ѧʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com