
����Ŀ��NaClO2��һ����Ҫ��ɱ����������Ҳ������Ư��֯��ȣ���һ�������������£��ش���������
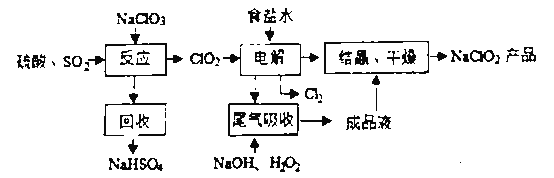
��1��NaClO2��Cl�Ļ��ϼ�Ϊ______________________.
��2��д������Ӧ������������ClO2�Ļ�ѧ����ʽ._______________________________��
��3�������������ʳ��ˮ�ɴ���ˮ���ƶ��ɡ����ξ���ʱ��Ϊ��ȥ���е�Mg 2+��Ca 2+��Ҫ������Լ��ֱ�Ϊ_____________________��______________________��
��4����β��������������������������ų�������ClO2�������շ�Ӧ![]() �����������������������֮��Ϊ_________________���ڱ��������44.8LO2��ת�Ƶ��ӵ����ʵ�����____.
�����������������������֮��Ϊ_________________���ڱ��������44.8LO2��ת�Ƶ��ӵ����ʵ�����____.
��5��˫��ˮ����ѧ��������ɫ��ѧ�Լ����ȿ��Ա��������ԣ��ֿ��Ա��ֻ�ԭ�ԡ���˫��ˮ�еμ����Ը��������Һ���������ݣ��Ϻ�ɫ��ȥ����֪Mn2+Ϊ��ɫ������������д�����ӷ���ʽ._____________________________��
���𰸡�+3 2NaClO3+SO2+H2SO4=2NaHSO4+2ClO2 NaOH��Һ Na2CO3��Һ 2��1 4mol ![]()
��������
��1����NaClO2��NaΪ+1�ۣ�OΪ-2�ۣ������������ϼ۵Ĵ�����Ϊ0����õ���
��2��NaClO3��SO2��H2SO4�ữ����������ClO2������NaClO2������������ԭ����ΪNaCl�����ղ���ΪNaHSO4��˵�������������ƣ��Ҳ���ClO2�����ݵ����غ��ԭ���غ���ƽ��д��ѧ����ʽ��
��3��ʳ����Һ�л���Mg2+ ��Ca2+���������ù���NaOH��Һ��ȥMg2+�����ù���Na2CO3��Һ��ȥCa2+��
��4������ͼʾ��֪�����ú��������������������Һ����ClO2������ΪClO2-����˷�Ӧ��ClO2Ϊ����������ԭ����ΪClO2-�����ϼ۴�+4�۽�Ϊ+3�ۣ�H2O2Ϊ��ԭ������������ΪO2��ÿĦ��H2O2�õ�2mol���ӣ����ݵ����غ��֪�������ͻ�ԭ�������ʵ���֮�ȣ�
��5����˫��ˮ�еμ����Ը��������Һ���������ݣ��Ϻ�ɫ��ȥ����֪Mn2+Ϊ��ɫ����˵��������ر���ԭ����Mn2+����˫��ˮ��������H2O2����HΪ+1�ۣ����ܱ�����������������ӦΪ������
����1����NaClO2��NaΪ+1�ۣ�OΪ-2�ۣ������������ϼ۵Ĵ�����Ϊ0���ɵ�Cl�Ļ��ϼ�Ϊ+3�ۣ�
�ʴ�Ϊ��+3��
��2��NaClO3��SO2��H2SO4�ữ����������ClO2������NaClO3������������ԭ����ΪClO2�����ղ���ΪNaHSO4��˵�������������ƣ��Ҳ���ClO2�����ݵ����غ��֪���˷�Ӧ�Ļ�ѧ����ʽΪ��2NaClO3+SO2+H2SO4=2NaHSO4+2ClO2��
�ʴ�Ϊ��2NaClO3+SO2+H2SO4=2NaHSO4+2ClO2��
��3��ʳ����Һ�л���Mg2+ ��Ca2+���������ù���NaOH��Һ��ȥMg2+�����ù���Na2CO3��Һ��ȥCa2+��
�ʴ�Ϊ��NaOH��Һ��Na2CO3��Һ��
��4������ͼʾ��֪�����ú��������������������Һ����ClO2������ΪClO2-����˷�Ӧ��ClO2Ϊ����������ԭ����ΪClO2-�����ϼ۴�+4�۽�Ϊ+3�ۣ�H2O2Ϊ��ԭ������������ΪO2��ÿĦ��H2O2�õ�2mol���ӣ����ݵ����غ��֪�������ͻ�ԭ�������ʵ���֮��Ϊ2��1�������44.8LO2�����ʵ���Ϊ2mol������ǰ��������֪ת�Ƶ�����Ϊ4mol��
�ʴ�Ϊ��2��1��4mol��
��5�����ݷ�����֪������ر���ԭ����Mn2+��˫��ˮ�������������������ݵ����غ��Ԫ���غ��֪���ӷ���ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊȷ��Na2CO3��NaHCO3�������Ʒ���������ȡ�ķݸ���Ʒ����ˮ��ֱ���μ�����ͬŨ������30.0 mL����ַ�Ӧ������CO2�����(������ɱ�״���µ������������CO2��ˮ�е��ܽ�)���±���
ʵ����� | �� | �� | �� | �� |
�������(mL) | 30.0 | 30.0 | 30.0 | 30.0 |
��Ʒ����(g) | 2.96 | 3.70 | 5.18 | 6.66 |
CO2���(mL) | 672 | 840 | 896 | 672 |
(1)��Ʒ�е����ʵ���֮��n(Na2CO3)��n(NaHCO3)��________��
(2)��������ʵ���Ũ��c(HCl)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ��̽��ij��ʯ���������ֶ�����Ԫ�أ�����ɺ����ʣ���֪��ʯ����һ���Ľᾧˮ��
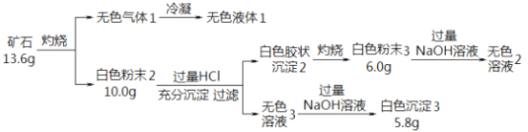
��1����ʯ�����Ԫ���� H��O ��_____�� ______����Ԫ�ط��ţ�����ѧʽΪ_____________��
��2����ɫ��ĩ3 ����NaOH��Һ�����ӷ���ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���ܴﵽʵ��Ŀ���ҷ��ϲ���Ҫ�����

A. I�������Ʊ������������Ŀ�ȼ��
B. II�����ڳ�ȥCO2�е�HCl
C. ����������һ�����ʵ���Ũ�ȵ�NaOH��Һ
D. ��IV�в����Ʊ��������������۲�����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨ̼���ƺ�̼�����ƻ����Ʒ��̼���Ƶ�������������ͨ�����ȷֽ�õ���![]() �������м���.ijͬѧ��Ƶ�ʵ��װ��ʾ��ͼ���£�
�������м���.ijͬѧ��Ƶ�ʵ��װ��ʾ��ͼ���£�

��ش�
��1��A�з�����Ӧ�Ļ�ѧ����ʽ��__________________________��
��2��װ��B�б�ˮ��������_________________________��
��3����ͬѧ��Ƶ�ʵ��װ�ô���ȱ�ݣ��Ľ����ʵ��װ�ü�ʵ������У��������ؿ���ʹ̼���Ƶ���������ƫ�ߵ���________________������ĸ����
A����Ʒ�ֽⲻ��ȫ
B������![]() ������̫�죬û�б���ʯ����ȫ����
������̫�죬û�б���ʯ����ȫ����
C����Ӧ��ȫ��ֹͣ���ȣ�ͨ������Ŀ���.
��4����C�еļ�ʯ�Ҹ�������![]() ���壬�����ʵ�鷽�����鷴Ӧ��C�й���ijɷ�___________��
���壬�����ʵ�鷽�����鷴Ӧ��C�й���ijɷ�___________��
��5��������Ʒ���Ƿ���NaHCO3����ѡ������װ�����ʵ�飬������±���

ѡ���װ��(����) | ʵ������ | ʵ����� |
��_______________________________ | ��_________________ | ��Ʒ�к�NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Ƿ�������ʱ���õ����������Խ��еĻ�����������ֱ��ǣ���
![]()
A.�����ˡ���ȡ������B.������������ȡ������
C.��ȡ�����ˡ���������D.���ˡ���������ȡ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��þ�����ֱ����Ũ�ȡ�������Ĺ���ϡ���ᷴӦ��������������(V)��ʱ��(t)�Ĺ�ϵ����ͼ��ʾ����Ӧ��þ������(����)

A. ���ʵ���֮��Ϊ3��2 B. ����֮��Ϊ3��2
C. Ħ������֮��Ϊ2��3 D. ʧȥ�ĵ�����֮��Ϊ8��9
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС��ģ��ʪ�������������ӡˢ��·�徭�ܽ⡢��ȡ�����Ȳ�������ͭ����ȡ���������̼�ͼ���£�

��֪ͭ����Һ��ͭԪ����[Cu(NH3)4]2+��ʽ���ڡ�
�����й�˵����ȷ����
A.��Ӧ����H2O2�������ǻ�ԭ���������ٵ������ǹ���
B.�����ں͢��õ�����Ҫ�������Ƿ�Һ©�������в����ڵ�Ŀ��������![]() ��ˮ�е��ܽ��
��ˮ�е��ܽ��
C.�������Ǽ�����������ۣ����ˣ������ݵ���Ҫ����������Ũ������ȴ�ᾧ������
D.�����п�ѭ��ʹ�õ��Լ���NH4Cl���л��ܼ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������A�Ǻϳ���Ȼ�ĵ��壬����ʽΪC5H8��A��һϵ�з�Ӧ����(���ַ�Ӧ������ȥ)��

��֪��
�ش��������⣺
(1)A�Ľṹ��ʽΪ_____________����ѧ������________________________��
(2)B�ķ���ʽΪ__________________________��
(3)�ڷ�Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
(4)�ٺ͢۵ķ�Ӧ���ͷֱ���___________��______________��
(5)CΪ�������������������Ǽ����ܵĻ�ѧ����ʽ��______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com