
NaNO2����ۺ�ʳ�����ƣ�������ζ������ʹ����ʳ�ж�����֪NaNO2�ܷ������·�Ӧ��2NaNO2��4HI===2NO����I2��2NaI��2H2O��
(1)������Ӧ����������____________��
(2)����������Ӧ������NaNO2��NaCl���塣��ѡ�õ������У���ˮ���ڵ⻯�ص�����ֽ���۵��ۡ��ܰơ���ʳ�ף�����Ϊ����ѡ�õ�������____________(�����)��
(3)ij����Һ�У�����2%��5%��NaNO2��ֱ���ŷŻ������Ⱦ�������Լ���ʹNaNO2ת��Ϊ�����������Ⱦ��N2����____________(����)��
A��NaCl B��NH4Cl C��HNO3 D��ŨH2SO4
(4)����ƽ���»�ѧ����ʽ��_______Al��_______NaNO3��_______NaOH===_______NaAlO2��_______N2����_________������Ӧ������ת��5 mol e���������ɱ�״����N2�����Ϊ________L��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�߶���9���¿���ѧ���������棩 ���ͣ�ѡ����
ֻ��һ���Լ���������Na2SO4��AlCl3��NH4Cl��MgSO4������Һ�������Լ���
A��HCl B��BaCl2 C��AgNO3 D��NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ������ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ɫ��Ӧ��ָ�� ��
A. ��ȼ����ȼ��ʱ����ʾ�Ļ�����ɫ
B. ����Ԫ��������ʱ������ɫ��Ӧ
C. ���ֽ��������ǵĻ���������ʱ����ʾ�Ļ�����ɫ
D. ��ɫ��Ӧ��һ�ֻ�ѧ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��������Ϊa��ij���ʵ���Һmg����������Ϊb�ĸ����ʵ���Һng��Ϻ�������pgˮ���õ�����Һÿ��������Ϊqg�����ʵ���Ũ��Ϊc�������ʵ���Է�������Ϊ���� ��
A�� B��
B��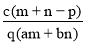
C��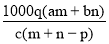 D��
D��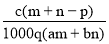
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�л���Ľṹ��ʽΪHCOOCH2CH===CH2���������еĹ�������( )
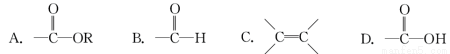
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ�ڵ��в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
Mg��AgCl�����һ���Ժ�ˮΪ�������Һ��ˮ�����ء���������������ǣ� ��
A��������ӦʽΪMg-2e-=Mg2+
B�������ᷢ������ӦMg+2H2O=Mg(OH)2+H2��
C��������ӦʽΪAg++e-=Ag
D����طŵ�ʱCl-��������Ǩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ�ڵ��в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£����и���������ָ����Һ��һ���ܴ���������ǣ� ��
A���μӼ����Ժ�ɫ����Һ�� K+�� NH4+��Cl?��SO42��
B���������ܷų���������Һ�� Na+�� NO3���� Cl?�� Ba2+
C��ʹ��ɫʯ����ֽ����ɫ����Һ�� Na+��ClO?��Cl?��S2?
D��c(H+)/c(OH?)=1013����Һ��Fe2+��Cl?��MnO4����SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�Ƹ��и�����ѧ�ڵ�һ�ε��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ijNaHCO3��Ʒ�л���������Na2CO3�����вⶨ����Ʒ���ȵ�ʵ�鷽���в��ܴﵽʵ��Ŀ�ĵ��ǣ� ��
A��ȡ��Ʒmg�����100ml��Һ��ȡ25.00mL����ƿ�У��μӼ���ʯ����Һ����Ũ��Ϊcmol/L�ı�����ζ����յ�ʱ����������Vml
B��ȡ��Ʒmg����ּ��ȣ���ȴ���������������Ϊn1g
C��ȡ��Ʒmg���μ�������BaCl2��Һ�����ˣ�ϴ�ӣ������������������Ϊn2g
D��ȡ��Ʒmg���μ�������Ba��OH��2���ˣ�ϴ�ӣ������������������Ϊn3g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������������彡����ɽϴ�Σ������
A����SO2Ư��ʳƷ
B����ʳ����ϴ��ˮƿ���ڱڸ��ŵ�ˮ��(CaCO3)
C������ˮ��ͨ������Cl2��������ɱ��
D����С�մ�(NaHCO3)��������������ͷ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com