


| “¼ |
| ”÷ |
| “¼ |
| ”÷ |
| ÅØĮņĖį |
| ”÷ |
| ÅØĮņĖį |
| ”÷ |


| NaOH“¼ |
| ”÷ |
| NaOH“¼ |
| ”÷ |
| “¼ |
| ”÷ |
| “¼ |
| ”÷ |
| ÅØĮņĖį |
| ”÷ |
 £»
£»| ÅØĮņĖį |
| ”÷ |
 £®
£®

 Č«ÓŵćĮ·µ„ŌŖ¼Ę»®ĻµĮŠ“š°ø
Č«ÓŵćĮ·µ„ŌŖ¼Ę»®ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
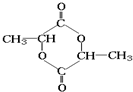
»ÆŗĻĪļAŹĒŹÆÓĶ»Æ¹¤µÄŅ»ÖÖÖŲŅŖŌĮĻ£¬ÓĆAŗĶĖ®ĆŗĘųĪŖŌĮĻ¾ĻĀĮŠĶ¾¾¶ŗĻ³É»ÆŗĻĪļD£Ø·Ö×ÓŹ½ĪŖC3H6O3£©”£


ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ½į¹¹¼ņŹ½£ŗ
A£ŗ____________,B£ŗ____________,C£ŗ____________,D£ŗ____________”£
£Ø2£©Öø³ö·“Ó¦¢ŚµÄ·“Ó¦ĄąŠĶ________________________”£
£Ø3£©Š“³ö·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½____________________________________________________”£
£Ø4£©·“Ó¦¢ÜµÄÄæµÄŹĒ____________________________________________________________”£
£Ø5£©»ÆŗĻĪļD”äŹĒDµÄŅ»ÖÖĶ¬·ÖŅģ¹¹Ģ壬Ėü×īŌē·¢ĻÖÓŚĖįÅ£ÄĢÖŠ£¬ŹĒČĖĢåÄŚĢĒĄą“śŠ»µÄÖŠ¼ä²śĪļ”£D”äŌŚÅØĮņĖį“ęŌŚµÄĢõ¼žĻĀ¼ÓČČ£¬¼ČæÉŅŌÉś³ÉÄÜŹ¹äåĖ®ĶŹÉ«µÄ»ÆŗĻĪļE£ØC3H4O2£©£¬ÓÖæÉŅŌÉś³ÉĮłŌ×Ó»·×“»ÆŗĻĪļF£ØC6H8O4£©”£Ēė·Ö±šŠ“³öD”äÉś³ÉEŗĶFµÄ»Æѧ·½³ĢŹ½£ŗ
D”䔜E£ŗ_____________________________________________________________________”£
D”䔜F£ŗ____________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆŗĻĪļAŹĒŹÆÓĶ»Æ¹¤µÄŅ»ÖÖÖŲŅŖŌĮĻ£¬ÓĆAŗĶĖ®ĆŗĘųĪŖŌĮĻ¾Ķ¼ĖłŹ¾Ķ¾¾¶ŗĻ³É»ÆŗĻĪļD(·Ö×ÓŹ½ĪŖC3H6O3)”£

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³öĻĀĮŠĪļÖŹµÄ½į¹¹¼ņŹ½£ŗA £»B £»C £»D ”£
(2)Öø³ö·“Ó¦¢ŚµÄ·“Ó¦ĄąŠĶ£ŗ ”£
(3)Š“³ö·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½£ŗ ”£
(4)·“Ó¦¢ÜµÄÄæµÄŹĒ ”£
(5)»ÆŗĻĪļD”äŹĒDµÄŅ»ÖÖĶ¬·ÖŅģ¹¹Ģ壬Ėü×īŌē·¢ĻÖÓŚĖįÅ£ÄĢÖŠ£¬ŹĒČĖĢåÄŚĢĒĄą“śŠ»µÄÖŠ¼ä²śĪļ”£D”äŌŚÅØĮņĖį“ęŌŚµÄĢõ¼žĻĀ¼ÓČČ£¬¼ČæÉŅŌÉś³ÉÄÜŹ¹äåĖ®ĶŹÉ«µÄ»ÆŗĻĪļE(C3H4O2)£¬ÓÖæÉŅŌÉś³ÉĮłŌ×Ó»·×“»ÆŗĻĪļF(C6H8O4)”£Ēė·Ö±šŠ“³öD”äÉś³ÉEŗĶFµÄ»Æѧ·½³ĢŹ½£ŗ
D”䔜E£ŗ ”£
D”䔜F£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģČżŠ£ÉĻŗ£ŹŠ£Øø“µ©”¢½»“󔢻ŖŹ¦“ó¶žø½ÖŠ£©øßČżĮŖæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø±¾Ģā¹²12·Ö£©
»ÆŗĻĪļAŹĒŹÆÓĶ»Æ¹¤µÄŅ»ÖÖÖŲŅŖŌĮĻ£¬ÓĆAŗĶĖ®ĆŗĘųĪŖŌĮĻ¾ĻĀĮŠĶ¾¾¶ŗĻ³É»ÆŗĻĪļD£Ø·Ö×ÓŹ½ĪŖC3H6O3£©”£
ŅŃÖŖ£ŗ




Ēė»Ų“šĻĀĮŠĪŹĢā£»
£Ø1£©Š“³öÓŠ¹ŲĪļÖŹµÄ½į¹¹¼ņŹ½£ŗA£ŗ £»B£ŗ £»C£ŗ £»D£ŗ ”£
£Ø2£©Öø³ö·“Ó¦¢ŻµÄ·“Ó¦ĄąŠĶ ”£
£Ø3£©Š“³ö·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ ”£
£Ø4£©·“Ó¦¢ÜµÄÄæµÄŹĒ ”£
»ÆŗĻĪļD”ÆŹĒDµÄŅ»ÖÖĶ¬·ÖŅģ¹¹Ģ壬Ėü×īŌē·¢ĻÖÓŚĖįÅ£ÄĢÖŠ£¬ŹĒČĖĢåÄŚĢĒĄą“śŠ»µÄÖŠ¼ä²śĪļ”£D”ÆŌŚÅØĮņĖį“ęŌŚµÄĢõ¼žĻĀ¼ÓČČ£¬¼ČæÉÉś³ÉÄÜŹ¹äåĖ®ĶŹÉ«µÄ»ÆŗĻĪļE£ØC3H4O2£©£¬ÓÖæÉÉś³ÉĮłŌ×Ó»·×“»ÆŗĻĪļF£ØC6H8O4£©”£
Ēė·Ö±šŠ“³öD”ÆÉś³ÉEŗĶFµÄ»Æѧ·½³ĢŹ½£ŗ
£Ø5£©D”Æ”śE£ŗ
£Ø6£©D”Æ”śF£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğȿŠ£ÉĻŗ£ŹŠ£©øßČżĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø±¾Ģā¹²12·Ö£©
»ÆŗĻĪļAŹĒŹÆÓĶ»Æ¹¤µÄŅ»ÖÖÖŲŅŖŌĮĻ£¬ÓĆAŗĶĖ®ĆŗĘųĪŖŌĮĻ¾ĻĀĮŠĶ¾¾¶ŗĻ³É»ÆŗĻĪļD£Ø·Ö×ÓŹ½ĪŖC3H6O3£©”£
ŅŃÖŖ£ŗ



Ēė»Ų“šĻĀĮŠĪŹĢā£»
£Ø1£©Š“³öÓŠ¹ŲĪļÖŹµÄ½į¹¹¼ņŹ½£ŗA£ŗ £»B£ŗ £»C£ŗ £»D£ŗ ”£
£Ø2£©Öø³ö·“Ó¦¢ŻµÄ·“Ó¦ĄąŠĶ ”£
£Ø3£©Š“³ö·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ ”£
£Ø4£©·“Ó¦¢ÜµÄÄæµÄŹĒ ”£
»ÆŗĻĪļD”ÆŹĒDµÄŅ»ÖÖĶ¬·ÖŅģ¹¹Ģ壬Ėü×īŌē·¢ĻÖÓŚĖįÅ£ÄĢÖŠ£¬ŹĒČĖĢåÄŚĢĒĄą“śŠ»µÄÖŠ¼ä²śĪļ”£D”ÆŌŚÅØĮņĖį“ęŌŚµÄĢõ¼žĻĀ¼ÓČČ£¬¼ČæÉÉś³ÉÄÜŹ¹äåĖ®ĶŹÉ«µÄ»ÆŗĻĪļE£ØC3H4O2£©£¬ÓÖæÉÉś³ÉĮłŌ×Ó»·×“»ÆŗĻĪļF£ØC6H8O4£©”£
Ēė·Ö±šŠ“³öD”ÆÉś³ÉEŗĶFµÄ»Æѧ·½³ĢŹ½£ŗ
£Ø5£©D”Æ”śE£ŗ
£Ø6£©D”Æ”śF£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com