



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ��Ŀ�� | ���� |
| A | ȷ��̼����Ԫ�طǽ�����ǿ�� | ��ͬ��ͬŨ��Na2CO3��Na2SiO3ˮ��Һ��pH |
| B | ��ȥ���л��еı��� | ����Һ�м���Ũ��ˮ,��ַ�Ӧ��,���� |
| C | ֤��SO2����Ư���� | ��SO2ͨ������KMnO4��Һ�� |
| D | ֤���������к���Ԫ�� | ���������еμ���AgNO3��Һ�ٵμ�ϡHNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
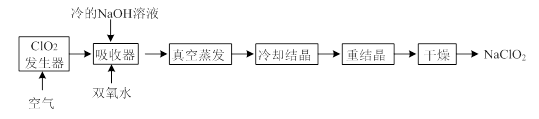
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����백ˮʱ���ɰ�ɫ����������ˮ����ʱ��ɫ������ʧ����ԭ��Һ��һ����Al3������ |
| B��������FeCl2��Һ��AlCl3��Һ��AgNO3��Һ����3�ִ���Һ�зֱ�μ�������ˮ |
| C���������ᣬ����ʹ����ʯ��ˮ����ǵ��������ɣ���ԭ��Һ��һ���д�����CO32-���� |
| D������BaCl2��Һ���ɰ�ɫ�������ټ���������������ܽ⣬��ԭ��Һ��һ����SO42-���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
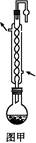
 ����,�������ϵ�֪һ���ϳ�·��:
����,�������ϵ�֪һ���ϳ�·��: CH2+CO+H2
CH2+CO+H2 CH3CH2CH2CHO
CH3CH2CH2CHO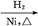 CH3CH2CH2CH2OH;
CH3CH2CH2CH2OH;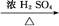 CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
 ����,����ѡ����ʵ��Լ��Ʊ���������ϩ,д����ѧ��Ӧ����ʽ:�� ,������������������������������
����,����ѡ����ʵ��Լ��Ʊ���������ϩ,д����ѧ��Ӧ����ʽ:�� ,������������������������������  ������Ʒ��Ϊ����1
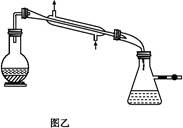
������Ʒ��Ϊ����1 ����,��С���������֪:��R��CHO+NaHSO3(����)
����,��С���������֪:��R��CHO+NaHSO3(����) RCH(OH)SO3Na��;�ڷе�:����34��,1
RCH(OH)SO3Na��;�ڷе�:����34��,1 ���� 118��,����Ƴ������ᴿ·��:
���� 118��,����Ƴ������ᴿ·��: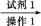 ��Һ
��Һ
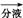 ���
���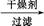 1
1 ����������
���������� ��Ʒ
��Ʒ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥCl2��������HCl���ɽ��������ͨ��ʢ�б���NaCl��Һ��ϴ��ƿ |
| B��������ڿ�����FeCl2��Һ�е���KSCN��Һ�������Fe��SCN��3Ѫ��ɫ���� |
| C����ij��Һ�е���ϡ�������ʹ����ʯ��ˮ����ǵ����壬����Һһ������CO32- |
| D��ʵ���ҿɲ��÷�Һ��ֱ�ӷ��������������Ҵ��Ļ��Һ |
�鿴�𰸺ͽ���>>
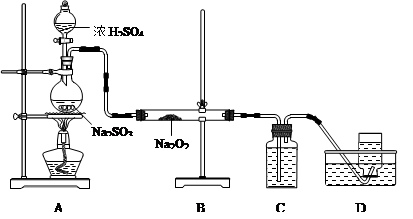
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
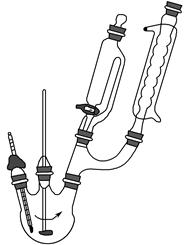
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��һ�������¿�����H2��ȥ�����л��е���ϩ |
| B���������Ȼ�̼������ȡ��ˮ�е��� |
| C���Ҵ�������������������ߵĻ��Һ���÷�Һ�ķ������� |
| D�����顢��ϩ�ͱ��ڹ�ҵ�϶���ͨ��ʯ�ͷ���õ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ���ᴿ������(����) | �����Լ� | ���뷽�� |
| A | NH3(H2O) | Ũ���� | ϴ�� |
| B | H2O(Br2) | CCl4 | ��Һ |
| C | KCl����(I2) | KOH��Һ | ���� |
| D | MgCl2��Һ(FeCl3) | NaOH��Һ | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com