
ŹÆÄ«ŌŚ²ÄĮĻĮģÓņÓŠÖŲŅŖÓ¦ÓĆ”£Ä³³õ¼¶ŹÆÄ«ÖŠŗ¬ SiO2£Ø7.8%£©”¢Al2O3(5.1%)”¢Fe2O3(3.1%)ŗĶMgO(0.5%)µČŌÓÖŹ”£Éč¼ĘµÄĢį“æŗĶ×ŪŗĻÓ¦ÓĆ¹¤ŅÕČēĻĀ£ŗ
SiO2£Ø7.8%£©”¢Al2O3(5.1%)”¢Fe2O3(3.1%)ŗĶMgO(0.5%)µČŌÓÖŹ”£Éč¼ĘµÄĢį“æŗĶ×ŪŗĻÓ¦ÓĆ¹¤ŅÕČēĻĀ£ŗ
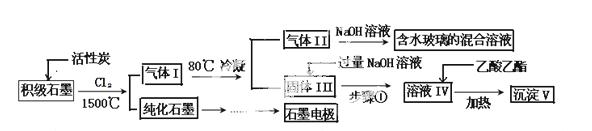
£Ø×¢£ŗSiCl4µÄ·ŠµćŹĒ 57.6ºC£¬½šŹōĀČ»ÆĪļµÄ·Šµć¾łøßÓŚ150ºC£©
57.6ºC£¬½šŹōĀČ»ÆĪļµÄ·Šµć¾łøßÓŚ150ºC£©
£Ø1£©Ļņ·“Ó¦Ę÷ÖŠĶØČėCl2Ē°£¬ŠčĶØŅ»¶ĪŹ±¼äµÄN2£¬Ö÷ŅŖÄæµÄŹĒ ”£
£Ø2£©øßĪĀ·“Ó¦ŗó£¬ŹÆÄ«ÖŠµÄŃõ»ÆĪļŌÓÖŹ¾ł×Ŗ±äĪŖĻąÓ¦µÄĀČ»ÆĪļ”£ĘųĢåIÖŠµÄĀČ»ÆĪļÖ÷ŅŖĪŖ ”£ÓÉĘųĢåII֊ijĪļÖŹµĆµ½Ė®²£Į§µÄ»Æ ѧ·½³ĢŹ½ĪŖ ”£
ѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©²½Öč¢ŁĪŖ£ŗ½Į°č”¢ ”£ĖłµĆČÜŅŗIVÖŠŅõĄė×ÓÓŠ ”£
£Ø4£©ÓÉČÜŅŗIVÉś³É³ĮµķVµÄ×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£100kg³õ¼¶ŹÆÄ«×ī¶ąæÉ»ńµĆVµÄÖŹĮæĪŖ kg”£
£Ø5£©ŹÆÄ«æÉÓĆÓŚ×ŌČ»Ė®ĢåÖŠĶ¼žµÄµē»Æѧ·ĄøÆ£¬Ķź³ÉĶ¼19·ĄøÆŹ¾ŅāĶ¼£¬²¢×÷ĻąÓ¦±ź×¢”£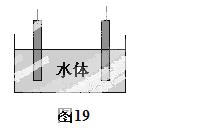
 ¶į¹ŚŃµĮ·µ„ŌŖĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
¶į¹ŚŃµĮ·µ„ŌŖĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø ŠĀĖ¼Ī¬Š”¹Ś¾ü100·Ö×÷Ņµ±¾ĻµĮŠ“š°ø
ŠĀĖ¼Ī¬Š”¹Ś¾ü100·Ö×÷Ņµ±¾ĻµĮŠ“š°ø ĆūŹ¦Öøµ¼Ņ»¾ķĶØĻµĮŠ“š°ø
ĆūŹ¦Öøµ¼Ņ»¾ķĶØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijӊ»śĪļn g£¬øś×ćĮ潚ŹōÄĘ·“Ӧɜ³ÉV L H2£¬ĮķČ”n gøĆÓŠ»śĪļÓė×ćĮæĢ¼ĖįĒāÄĘ×÷ÓĆÉś³ÉV L CO2(ĻąĶ¬×“æöĻĀ)£¬øĆÓŠ»śĪļ·Ö×ÓÖŠŗ¬ÓŠµÄ¹ŁÄÜĶÅĪŖ(””””)
A£®ŗ¬Ņ»øöōČ»łŗĶŅ»øöōĒ»ł
B£®ŗ¬Į½øöōČ»ł
C£®Ö»ŗ¬Ņ»øöōČ»ł
D£®ŗ¬Į½øöōĒ»ł
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĖłŹ¾ŹĒŹµŃéŹŅÕōĮóŹÆÓĶµÄŹµŃé×°ÖĆ£¬ŹµŃéŠčŅŖŹÕ¼Æ60”«150 ”ęŗĶ150”«300 ”ęµÄĮó·Ö”£
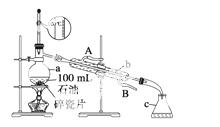
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŅĒĘ÷aµÄ¹ęøńŗĶĆū³Ę____________£¬ŅĒĘ÷b”¢cµÄĆū³Ę£ŗb__ ______£¬c________”£
______£¬c________”£
(2)ŹµŃéĖłŃ”ĪĀ¶Č¼ĘŹĒ“ķĪóµÄ£¬ÕżČ·µÄŃ”ŌńÓ¦øĆŹĒ____________________________”£
(3)ŹµŃ鏱ŌŚŅĒĘ÷aÖŠ¼ÓČėĮĖÉŁĮæĖé“Éʬ£¬ĘäÄæµÄŹĒ__________________________”£
(4)ŹµŃ鏱ŌŚŅĒĘ÷bÖŠĶØČėĄäČ“Ė®£¬ĒėÖøĆ÷ĄäČ“Ė®µÄĮ÷Ļņ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
CŹĒŅ»ÖÖŗĻ³ÉŹ÷Ö¬£¬ÓĆÓŚÖʱøĖÜĮĻŗĶŗĻ³ÉĻĖĪ¬£¬DŹĒŅ»ÖÖÖ²ĪļÉś³¤µ÷½Ś¼Į£¬ÓĆĖüæÉŅŌ“ߏģ¹ūŹµ”£øł¾ŻŅŌĻĀ»Æѧ·“Ó¦æņĶ¼ĢīæÕ£ŗ
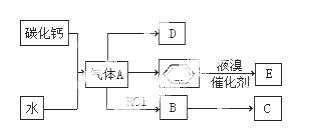
£Ø1£©Š“³öAµÄµē×ÓŹ½ £»DµÄ×ī¼ņŹ½ £»
£»DµÄ×ī¼ņŹ½ £»
£Ø2£©Š“³öĢ¼»ÆøĘÓėĖ®
 ·“Ó¦ÖĘČ”AµÄ»Æѧ
·“Ó¦ÖĘČ”AµÄ»Æѧ ·½³ĢŹ½ £»±½ŗĶŅŗäå·“Ó¦Éś³ÉEµÄ»Æѧ·½³ĢŹ½ £¬Ęä·“Ó¦ĄąŠĶĪŖ ”£B”śCµÄ»Æѧ·½³ĢŹ½ £¬Ęä·“Ó¦ĄąŠĶĪŖ ”£
·½³ĢŹ½ £»±½ŗĶŅŗäå·“Ó¦Éś³ÉEµÄ»Æѧ·½³ĢŹ½ £¬Ęä·“Ó¦ĄąŠĶĪŖ ”£B”śCµÄ»Æѧ·½³ĢŹ½ £¬Ęä·“Ó¦ĄąŠĶĪŖ ”£
£Ø3£©D»¹æÉŅŌÓĆŹÆĄÆÓĶÖĘČ”£¬DŌŚŅ»¶ØĢõ¼žĻĀ“ęŌŚČēĻĀ×Ŗ»Æ¹ŲĻµ£ØŹÆĄÆÓĶŗ¬17øöĢ¼Ō×ÓŅŌÉĻµÄŅŗĢ¬ĶéĢž£¬²æ·Ö·“Ó¦Ģõ¼ž”¢²śĪļ±»Ź”ĀŌ£©£¬GŹĒŅ»ÖÖĖįŠŌĪļÖŹ£¬HŹĒ¾ßÓŠ¹ūĻćĘųĪ¶µÄĢžµÄŃÜÉśĪļ”£
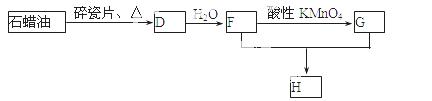
a£®¹¤ŅµÉĻ£¬ÓÉŹÆÓĶ»ńµĆĘūÓĶ”¢ĆŗÓĶ”¢ŹÆĄÆÓĶµČ³É·ŻµÄ·½·ØŹĒ £»
b£®D”śFµÄ»Æѧ·½³ĢŹ½ 
 £¬Ęä·“Ó¦ĄąŠĶŹĒ ”£
£¬Ęä·“Ó¦ĄąŠĶŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
LiPF6ŹĒļ®Ąė×Óµē³ŲÖŠ¹ć·ŗÓ¦ÓƵĵē½āÖŹ”£Ä³¹¤³§ÓĆLiF”¢PCl5ĪŖŌĮĻ£¬µĶĪĀ·“Ó¦ÖʱøLiPF6£¬ĘäĮ÷³ĢČēĻĀ£ŗ
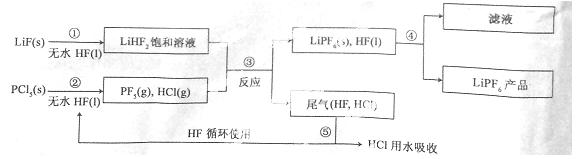
ŅŃÖŖ£ŗHClµÄ·ŠµćŹĒ£85.0 ”ę£¬HFµÄ·ŠµćŹĒ19.5 ”ę”£
£Ø1£©µŚ¢Ł²½·“Ó¦ÖŠĪŽĖ®HFµÄ×÷ÓĆŹĒ ”¢ ”£·“Ó¦Éč±ø²»ÄÜÓĆ²£Į§²ÄÖŹµÄŌŅņŹĒ (ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)”£ĪŽĖ®HFÓŠøÆŹ“ŠŌŗĶ¶¾ŠŌ£¬¹¤³§°²Č«ŹÖ²įĢįŹ¾£ŗČē¹ū²»Š”ŠÄ½«HFÕ“µ½Ę¤·ōÉĻ£¬æÉĮ¢¼“ÓĆ2%µÄ ČÜŅŗ³åĻ“”£
£Ø2£©øĆĮ÷³ĢŠčŌŚĪŽĖ®Ģõ¼žĻĀ½ųŠŠ£¬µŚ¢Ū²½·“Ó¦ÖŠPCl5¼«Ņ×Ė®½ā£¬Ęä²śĪļĪŖĮ½ÖÖĖį£¬Š“³öPF5Ė®½āµÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©µŚ¢Ü²½·ÖĄė²ÉÓƵķ½·ØŹĒ £»µŚ¢Ż²½·ÖĄėĪ²ĘųÖŠHF”¢HCl²ÉÓƵķ½·ØŹĒ ”£
£Ø4£©LiPF6²śĘ·ÖŠĶس£»ģÓŠÉŁĮæLiF”£Č”ѳʷwg£¬²āµĆLiµÄĪļÖŹµÄĮæĪŖnmol£¬ŌņøĆѳʷ֊LiPF6µÄĪļÖŹµÄĮæĪŖ mol(ÓĆŗ¬ÓŠw”¢nµÄ“śŹżŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
·Ļ¾ÉÓ²ÖŹŗĻ½šµ¶¾ßÖŠŗ¬Ģ¼»ÆĪŁ£ØWC£©”¢½šŹōīÜ£ØCo£©¼°ÉŁĮæŌÓÖŹĢś£¬ĄūÓƵē½ā·ØæÉ»ŲŹÕWCŗĶCo”£¹¤ŅÕĮ÷³Ģ¼ņĶ¼ČēĻĀ£ŗ

£Ø1£©½āŹ±·Ļ¾Éµ¶¾ß×öŃō¼«£¬²»ŠāøÖ×öŅõ¼«£¬HClČÜŅŗĪŖµē½āŅŗ”£Ņõ¼«Ö÷ŅŖµÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø2£©¾»»Æ²½ÖčĖłµĆĀĖ±żµÄÖ÷ŅŖ³É·ÖŹĒ ”£»ŲŹÕµÄĻ“µÓŅŗ“śĢęĖ®ÅäÖʵē½āŅŗ£¬ÄæµÄŹĒ»ŲŹÕĄūÓĆĘäÖŠµÄ ”£
¢ĒČÜŅŗIµÄÖ÷ŅŖ³É·ÖŹĒ  ”£Ļ“µÓCoC2O4²»³ä·Ö¶Ō×īÖÕ²śĘ·“æ¶Č²¢ĪŽĆ÷ĻŌÓ°Ļģ£¬µ«±ŗÉÕŹ±»įŌģ³É»·¾³ĪŪČ¾£¬ŌŅņŹĒ ”£
”£Ļ“µÓCoC2O4²»³ä·Ö¶Ō×īÖÕ²śĘ·“æ¶Č²¢ĪŽĆ÷ĻŌÓ°Ļģ£¬µ«±ŗÉÕŹ±»įŌģ³É»·¾³ĪŪČ¾£¬ŌŅņŹĒ ”£
¢Č½«Co2O3»¹Ō³ÉCo·ŪµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧŹµŃéŹŅĶس£ÓĆ“ÖŠæŗĶĻ”ĮņĖį·“Ó¦ÖĘĒāĘų£¬Ņņ“ĖŌŚÖĘĒā·ĻŅŗÖŠŗ¬ÓŠ“óĮæµÄĮņĖįŠæ”£Ķ¬Ź±£¬ÓÉÓŚ“ÖŠæÖŠ»¹ŗ¬ÓŠĢśµČŌÓÖŹ£¬Ź¹µĆČÜŅŗÖŠ»ģÓŠŅ»¶ØĮæµÄĮņĖįŃĒĢś£¬ĪŖĮĖ³ä·ÖĄūÓĆÖĘĒā·ĻŅŗ£¬³£ÓĆĘäÖʱøš©·Æ(ZnSO4·7H2O)”£Ä³Š£»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ŅŌÖĘĒāĘųµÄ·ĻŅŗĪŖŌĮĻĄ“ÖĘČ”š©·Æ”£Öʱøš©·ÆµÄŹµŃéĮ÷³ĢČēĻĀĶ¼ĖłŹ¾”£

ŅŃÖŖ£ŗæŖŹ¼Éś³ÉĒāŃõ»ÆĪļ³Įµķµ½³ĮµķĶźČ«µÄpH·¶Ī§·Ö±šĪŖFe(OH)3£ŗ2.7”«3.7£»Fe(OH)2£ŗ7.6”«9.6£»Zn(OH)2£ŗ5.7”«8.0£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¼ÓČėµÄŹŌ¼Į¢Ł£¬¹©Ń”ŌńŹ¹ÓƵÄÓŠ£ŗ°±Ė®”¢NaClOČÜŅŗ”¢20%µÄH2O2”¢ÅØĮņĖį”¢ÅØĻõĖįµČ£¬×īŗĆŃ”ÓĆ________£¬ĘäĄķÓÉŹĒ
______________________________________________________________________ӣ
(2)¼ÓČėµÄŹŌ¼Į¢Ś£ŗ¹©Ń”ŌńŹ¹ÓƵÄÓŠ£ŗa.Zn·Ū”¢b.ZnO”¢c.Zn(OH)2”¢d.ZnCO3”¢e.ZnSO4µČ£¬æÉŃ”ÓĆ__________”£
(3)“Ó¾§Ģå1”ś¾§Ģå2£¬øĆ¹ż³ĢµÄĆū³ĘŹĒ__________”£
(4)ŌŚµĆµ½š©·ÆŹ±£¬Ļņ¾§ĢåÖŠ¼ÓČėÉŁĮæ¾Ę¾«Ļ“µÓ¶ų²»ÓĆĖ®µÄŌŅņŹĒ_____________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄūĆŹĻ©ŹĒŅ»ÖÖŹ³ÓĆĻćĮĻ£¬Ęä½į¹¹¼ņŹ½ČēĶ¼ĖłŹ¾”£ĻĀĮŠÓŠ¹ŲÄūĆŹĻ©µÄ·ÖĪöÕżČ·µÄŹĒ(””””)
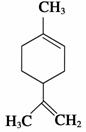 A£®ĖüµÄŅ»ĀČ“śĪļÓŠ6ÖÖ
A£®ĖüµÄŅ»ĀČ“śĪļÓŠ6ÖÖ
B£®ĖüµÄ·Ö×ÓÖŠĖłÓŠµÄĢ¼Ō×ÓŅ»¶ØŌŚĶ¬Ņ»Ę½ĆęÉĻ
C£®ĖüŗĶ¶”»ł±½(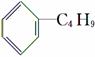 )»„ĪŖĶ¬·ÖŅģ¹¹Ģå
)»„ĪŖĶ¬·ÖŅģ¹¹Ģå
D£®Ņ»¶ØĢõ¼žĻĀ£¬Ėü·Ö±šæÉŅŌ·¢Éś¼Ó³É”¢Č”“ś”¢Ńõ»Æ”¢»¹ŌµČ·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖµŖŌŖĖŲŗĶŃõŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļÖŠ µŖŌŖĖŲŗĶŃõŌŖĖŲµÄÖŹĮæ±ČĪŖ7:16Ęä»ÆѧŹ½æÉÄÜĪŖ £Ø £© A NO B NO2 C N2O D N2O5
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com