
 ��
�� ���� ��1��C��O��NԪ�ض��ǵڶ����ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�
��2�����ݼ۲���ӶԻ�������ȷ������ԭ���ӻ����ͣ��۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ��������ۻ������γɵľ���Ϊ���Ӿ��壻
��3�����ݼ۲���ӶԻ������ۿ�֪���ַ��Ӿ���ȡsp3�ӻ���������ӵ��ӻ�������µ��Ӷԣ���H2O���������Թ¶Ե��ӣ��¶Ե�����ɼ����ӵ��ų�������С��
��4��Ԫ��M��������NH4+����������������������ͬ��M������������Ϊ11��������Ϊ10��MΪ��Ԫ�أ�
��� �⣺��1��C��O��NԪ�ض��ǵڶ����ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������N��O��C��
�ʴ�Ϊ��N��O��C��
��2��H2O������Oԭ�Ӽ۲���ӶԸ���=2+$\frac{6-2��1}{2}$=4������Oԭ�Ӳ���sp3�ӻ������ӿռ乹�������������Σ������������µ��Ӷԣ����Է��ӹ���ΪV�Σ�H2OΪ���ۻ�����γɷ��Ӿ��壬
�ʴ�Ϊ��V�Σ����Ӿ��壻
��3��CH4��̼ԭ�Ӽ۲���ӶԸ���=4+$\frac{4-4}{2}$=4��Ϊsp3�ӻ��������µ��Ӷԣ����Կռ乹�������������Σ������Ϊ109��28�䣬H2O����ԭ��Oԭ�ӳ�2���Ҽ�������$\frac{6-1��2}{2}$=2�Թ¶Ե��ӣ��۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ���=2+2=4���ӻ������Ϊ4����ȡsp3�ӻ���H2O���ӵ����幹����V�Σ������Ϊ104.5�㣬������ӵ��ӻ�������µ��Ӷԣ���H2O���������Թ¶Ե��ӣ��¶Ե�����ɼ����ӵ��ų�������С������H2O�ļ���С��CH4��
�ʴ�Ϊ���������ַ��Ӿ���ȡsp3�ӻ���������ӵ��ӻ�������µ��Ӷԣ���H2O���������Թ¶Ե��ӣ��¶Ե�����ɼ����ӵ��ų�������С��
��4��N��������Ϊ7��H��������Ϊ1��NH4+�к���10�����ӣ�11�����ӣ�Ԫ��M��������NH4+����������������������ͬ��M������������Ϊ11��������Ϊ10��MΪ��Ԫ�أ���M��ԭ�ӽṹʾ��ͼΪ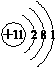
�ʴ�Ϊ��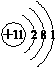
���� ������Ҫ�����˵�һ�����ܡ�ԭ���ӻ����͡�ԭ�ӽṹ��֪ʶ����Ŀ�Ѷ��еȣ�ע��۲���ӶԻ�����������Ӧ�ã�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | BCl3 | B�� | H2S | C�� | NCl3 | D�� | SF6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �缫a��װ���������� | |
| B�� | װ����ĤA�������ӽ���Ĥ | |
| C�� | �缫b�ĵ缫��Ӧʽ��2NO3-+10e-+12H+�TN2��+6H2O | |
| D�� | ���л����ʾΪC4H8O2��ÿת��10mol���ӣ��缫a�ϲ���22.4LCO2����״���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
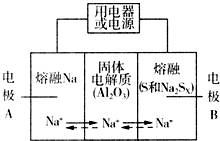 �����������ڽ���Na������S�Ͷ����ƣ�Na2SX���ֱ���Ϊ�����缫�ķ�Ӧ������Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ��䷴Ӧԭ����ͼ��ʾ��
�����������ڽ���Na������S�Ͷ����ƣ�Na2SX���ֱ���Ϊ�����缫�ķ�Ӧ������Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ��䷴Ӧԭ����ͼ��ʾ��| ���� | Na | S | Al2O3 |
| �۵�/�� | 97.8 | 115 | 2050 |
| �е�/�� | 892 | 444.6 | 2980 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | O.1mol/LKHC03��Һ��O��1mol/L KOH��Һ�������ϣ�������Һ�У�c��K+����c��CO32-����c��HCO3-����c��OH-�� | |
| B�� | pH=1��NaHSO4��Һ��c��H+���Tc��SO42-��+c��OH-�� | |
| C�� | 20ml O��lmol/L CH3COONa��Һ��lOmLO��lmol/L HCl��Һ��Ϻ���Һ�����ԣ�������Һ�У�C��Cl-������CH3COO-����c��H+����c��CH3COOH�� | |
| D�� | pH=2��H2C2O4��Һ��pH=l2��NaOH��Һ���������ϣ�c��Na+��+c��H+��=c��OH-��+c��HC2O4-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| X | |
| Y | Z |
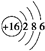 ��U2X�ĵ���ʽ
��U2X�ĵ���ʽ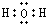
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | A | B | C | D |
| �������� | �⻯�� | �ɱ� | ʯī | �� |
| ������ ������ | ���������� | ���� | ԭ�� | ���� |
| ���Ӽ� ������ | ���Ӽ� | ���Ӽ� ������ | ���ۼ� | ���Ӽ� ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ������顢������ | B�� | �������Ȼ�̼����ϩ | ||
| C�� | �����ױ��������� | D�� | �״����������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
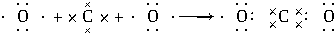 ���õ���ʽ��ʾC��D��Ԫ���γɻ�ѧ���Ĺ��̣�
���õ���ʽ��ʾC��D��Ԫ���γɻ�ѧ���Ĺ��̣�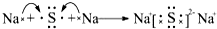 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com