
 N2O4����Ӧ�Ƿ��ȷ�Ӧ����Ӧǰ�����������С���Է�Ӧ����Ϊ������ѹǿ�����¶������ڷ�Ӧ������У�
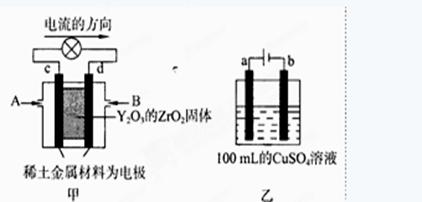
N2O4����Ӧ�Ƿ��ȷ�Ӧ����Ӧǰ�����������С���Է�Ӧ����Ϊ������ѹǿ�����¶������ڷ�Ӧ������У� 2Cu+O2��+2H2SO4�����Դ����������Ϊ��������Һ�� �����������ǵ��ӷ���������Ӧ���缫��ӦΪ��4OH��-4e��=2H2O+O2������a�缫����56mL����״��������Ϊ���������ʵ���Ϊ0.0025mol�������������������ʵ���Ϊ0.01mol����Һ���������������ʵ���Ϊ0.01mol��c��H����=
2Cu+O2��+2H2SO4�����Դ����������Ϊ��������Һ�� �����������ǵ��ӷ���������Ӧ���缫��ӦΪ��4OH��-4e��=2H2O+O2������a�缫����56mL����״��������Ϊ���������ʵ���Ϊ0.0025mol�������������������ʵ���Ϊ0.01mol����Һ���������������ʵ���Ϊ0.01mol��c��H����=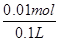 =0.1mol��L��1��PH=-lg0.1=1����������Һ��������CuSO4��Һÿ��ʧ2��Cuԭ�ӣ�����ʧ2�� Oԭ�ӣ��൱����ʧһ��CuO��Ϊ��ʹCuSO4��Һ���ָ�ԭŨ�ȣ�Ӧ����CuO��Ҳ���Լ���CuCO3�����ϻָ���ҺŨ�ȵĶ�����ϵ�������ܼ���Cu��OH��2��Cu2��OH��2CO3����ΪCuCO3+H2SO4
=0.1mol��L��1��PH=-lg0.1=1����������Һ��������CuSO4��Һÿ��ʧ2��Cuԭ�ӣ�����ʧ2�� Oԭ�ӣ��൱����ʧһ��CuO��Ϊ��ʹCuSO4��Һ���ָ�ԭŨ�ȣ�Ӧ����CuO��Ҳ���Լ���CuCO3�����ϻָ���ҺŨ�ȵĶ�����ϵ�������ܼ���Cu��OH��2��Cu2��OH��2CO3����ΪCuCO3+H2SO4 CuSO4+CO2��+H2O���൱�ڼ�CuO����Cu��OH��2+H2SO4
CuSO4+CO2��+H2O���൱�ڼ�CuO����Cu��OH��2+H2SO4 CuSO4+2H2O��Cu2��OH��2CO3+2H2SO4=2CuSO4 +CO2��+3H2O�������������������ˮ��ѡac��
CuSO4+2H2O��Cu2��OH��2CO3+2H2SO4=2CuSO4 +CO2��+3H2O�������������������ˮ��ѡac��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 cC(��)+dD(��)����H��Q������ͼ�ش�
cC(��)+dD(��)����H��Q������ͼ�ش�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


 4CO(g)+BaS(s) ��H1 = +571.2kJ/mol ��
4CO(g)+BaS(s) ��H1 = +571.2kJ/mol �� 2CO(g) ��H2 = +172.5kJ/mol ��
2CO(g) ��H2 = +172.5kJ/mol �� 2CO2(g) + BaS(s) ��H3 =
2CO2(g) + BaS(s) ��H3 = �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��21.44kJ | B��600.20kJ | C��21435.71kJ | D��1965.10kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2912 kJ | B��2953 kJ | C��3236 kJ | D��3867 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��+1533��8 | B���� 1533��8 | C��+295��4 | D����295��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

 CO(NH2)2(l)+H2O(g)��
CO(NH2)2(l)+H2O(g)��| A�����NH3��Ũ�� | B������ѹǿ |
| C����ʱת�����ɵ����� | D��ʹ�ø���Ч�Ĵ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com