
Ēėøł¾ŻĻĀĶ¼»Ų“š£ŗ
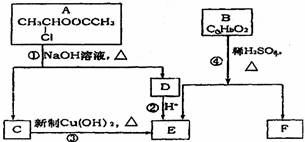
ŅŃÖŖ£ŗŅ»øöĢ¼Ō×ÓÉĻĮ¬ÓŠĮ½øöōĒ»łŹ±£¬Ņ×·¢ÉśĻĀĮŠ×Ŗ»Æ£ŗ
![]()
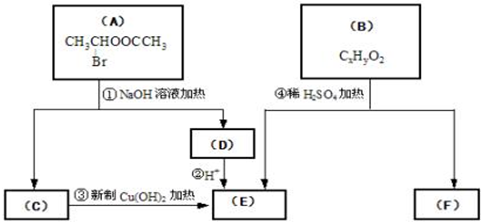
£Ø1£©EÖŠŗ¬ÓŠµÄ¹ŁÄÜĶŵÄĆū³ĘŹĒ ”£
£Ø2£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø3£©ŅŃÖŖBµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ162£¬ĘäĶźČ«Č¼ÉյIJśĪļÖŠn£ØCO2£©: n£ØH2O£©=2:1£¬ŌņBµÄ·Ö×ÓŹ½ĪŖ ”£
£Ø4£©FŹĒøß·Ö×Ó¹ā×č¼ĮÉś²śµÄÖ÷ŅŖŌĮĻ”£F¾ßÓŠČēĻĀĢŲµć£ŗ
¢ŁÄÜøśFeCl3ČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦£» ¢ŚÄÜ·¢Éś¼Ó¾Ū·“Ó¦£»
¢Ū±½»·ÉĻµÄŅ»ĀČ“śĪļÖ»ÓŠĮ½ÖÖ”£
FŌŚŅ»¶ØĢõ¼žĻĀ·¢Éś¼Ó¾Ū·“Ó¦µÄ»Æѧ·½³ĢĪŖ ”£
£Ø5£©»ÆŗĻĪļGŹĒFµÄĶ¬·ÖŅģ¹¹Ģ壬ŹōÓŚ·¼Ļć×å»ÆŗĻĪļ£¬ÄÜ·¢ÉśŅų¾µ·“Ó¦”£GÓŠ¶ąÖÖ½į¹¹£¬Š“³öĘäÖŠŅ»ÖֵĽį¹¹¼ņŹ½ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ











²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
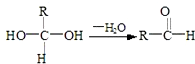
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010”Ŗ2011ѧğÕć½Ź”ĪĀ֯֊ѧøßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
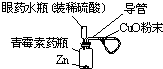
(12·Ö) µē»ÆѧŌĄķŌŚ»Æѧ¹¤ŅµÖŠÓŠ¹ć·ŗÓ¦ÓĆ”£Ēėøł¾ŻĻĀĶ¼»Ų“šÓŠ¹ŲĪŹĢā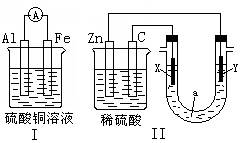
£Ø1£©×°ÖĆIÖŠÄÜĮæµÄ×Ŗ»ÆŠĪŹ½ŹĒ ”£µ¼ĻßÉĻµē×Ó¶ØĻņŅĘ¶Æ·½Ļņ“Ó ¼«µ½ ¼«”£Ęäµē¼«·“Ó¦Ź½ ”£
£Ø2£©×°ÖĆIIÖŠČōX”¢Y¶¼ŹĒ¶čŠŌµē¼«£¬aŹĒCuCl2ČÜŅŗ”£Ōņ·¢ÉśŃõ»Æ·“Ó¦µÄµē¼«ŹĒ ŗĶ ”£¼ģŃéXµē¼«·“Ó¦²śĪļµÄ·½·ØŹĒ ”£
ČōaŹĒ±„ŗĶNaClČÜŅŗ£ØĀČ¼ī¹¤ŅµÉś²śŌĄķ£©£¬µē¼«²ÄĮĻŹĒŹÆÄ«µē¼«ŗĶĢśµē¼«”£ŹµŃéæŖŹ¼Ź±£¬Ķ¬Ź±ŌŚĮ½¼«ø÷µĪČė¼øµĪ·ÓĢŖŹŌŅŗ£¬Ōņ £ØĢī”°ŹÆÄ«»ņĢś”±£©ø½½üČÜŅŗĻȱäŗģ£¬Ęäµē¼«·“Ó¦Ź½ ”£
£Ø3£©µ±×°ÖĆIŗĶ×°ÖĆIIµÄµēĀ·ÖŠ¾ł×ŖŅĘ0.2molµē×ÓŹ±£¬Ōņ×°ÖĆIÖŠĢśµē¼«ÖŹĮæµÄ±ä»Æ
g£ØĢīŠ“”°Ōö¼Ó”±»ņ”°¼õÉŁ”±¼°¾ßĢåÖŹĮ棩£¬×°ÖĆIIÖŠŹÕ¼Æµ½µÄĘųĢå¹²ÓŠ L£Ø±ź×¼×“æöĻĀ£©£ØŅŌ×°ÖĆIIÖŠX”¢YŹĒ¶čŠŌµē¼«£¬aŹĒCuCl2ČÜŅŗ¼ĘĖć£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com