
| ²ā¶ØŠņŗÅ | “ż²āČÜŅŗµÄĢå»ż£ØmL£© | ĖłŗÄŃĪĖį±ź×¼ŅŗµÄĢå»ż£ØmL£© | |
| µĪ¶ØĒ°¶ĮŹż | µĪ¶Øŗó¶ĮŹż | ||
| 1 | 20.00 | 0.50 | 20.78 |
| 2 | 20.00 | 1.20 | 21.32 |
·ÖĪö £Ø1£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗŠčŅŖŅĒĘ÷£ŗČŻĮæĘ攢½ŗĶ·µĪ¹Ü”¢ÉÕ±”¢²£Į§°ō”¢ĢģĘ½”¢Ņ©³×µČ£»
£Ø2£©¢ŁĖįŠŌČÜŅŗÓ¦ÓĆĖįŹ½µĪ¶Ø¹ÜŹ¢·Å£»¢ŚµĪ¶ØŹ±ŃŪ¾¦×¢ŹÓ׶ŠĪĘæÖŠŃÕÉ«±ä»Æ£»
£Ø3£©ĻČĖć³öĮ½“ĪĻūŗÄŃĪĖįµÄĘ½¾łĢå»ż£¬Č»ŗóĒó³öĒāŃõ»ÆÄʵÄĪļÖŹµÄĮ棬ŌŁ¼ĘĖćÉÕ¼īѳʷµÄ“æ¶Č£»
£Ø4£©¢ŁÓĆÕōĮóĖ®³åĻ“׶ŠĪĘ棬²»Ó°Ļģ“ż²āĪļµÄ×ÜĪļÖŹµÄĮ棻
¢ŚµĪ¶Ø¹ż³ĢÖŠ²»É÷½«ĖįµĪŌŚĘæĶā£¬ĻūŗÄŃĪĖįµÄĢå»żĘ«¶ą£»
¢ŪµĪ¶ØĒ°ŃöŹÓ”¢µĪ¶Øŗóø©ŹÓ£¬ĻūŗÄŃĪĖįĢå»żĘ«Š”£»
¢ÜĪ“ÓƱź×¼ŅŗČóĻ“µĪ¶Ø¹Ü£¬ĻūŗÄŃĪĖįĢå»żĘ«“ó£®
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘ1000mLŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗŠčŅŖŅĒĘ÷£ŗ1000mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü”¢ÉÕ±”¢²£Į§°ō”¢ĢģĘ½”¢Ņ©³×£¬ĖłŅŌÅäÖĘ“ż²āŅŗ»¹ŠčŅŖµÄ²£Į§ŅĒĘ÷ÓŠ1000mLČŻĮæĘ攢²£Į§°ō”¢½ŗĶ·µĪ¹Ü£¬ÉÕ±£»
¹Ź“š°øĪŖ£ŗ1000mLČŻĮæĘ棻
£Ø2£©¢ŁŹ¢×°0.1000mol•L-1ŃĪĖį±ź×¼ŅŗÓ¦øĆŹ¹ÓĆĖįŹ½µĪ¶Ø¹Ü£»¢ŚµĪ¶ØŹ±Ė«ŃŪӦעŅā¹Ū²ģ׶ŠĪĘæÄŚČÜŅŗŃÕÉ«µÄ±ä»Æ£»
¹Ź“š°øĪŖ£ŗĖį£»
¢ŚµĪ¶ØŹ±Ė«ŃŪӦעŅā¹Ū²ģ׶ŠĪĘæÄŚČÜŅŗŃÕÉ«µÄ±ä»Æ£¬¹Ź“š°øĪŖ£ŗ×¢Ņā¹Ū²ģ׶ŠĪĘæÄŚČÜŅŗŃÕÉ«µÄ±ä»Æ£»
£Ø3£©Į½“ĪĻūŗÄŃĪĖįĢå»ż·Ö±šĪŖ£ŗ20.28ml”¢20.12ml£¬ĖłŅŌĻūŗÄŃĪĖįµÄĘ½¾łĢå»żĪŖ20.20mL£¬20.00mLĒāŃõ»ÆÄĘČÜŅŗÖŠn£ØNaOH£©=n£ØHCl£©=0.10mol/L”Į0.0202L=0.00202mol£¬ĖłŅŌ1000mL“ż²āČÜŅŗŗ¬ÓŠm£ØÉÕ¼ī£©ØT0.00202mol”Į50”Į40g/mol=4.02g£¬ĖłŅŌÉÕ¼īµÄ“æ¶Č¦Ų£ØÉÕ¼ī£©=$\frac{4.02g}{5.0g}$”Į100%=80.4%£»
¹Ź“š°øĪŖ£ŗ80.4%£»
£Ø4£©¢Ł³åĻ“׶ŠĪĘ棬²»Ó°Ļģ“ż²āĪļµÄ×ÜĪļÖŹµÄĮ棬²»»įÓ°Ļģ²ā¶Ø½į¹ū£¬¹Ź“š°øĪŖ£ŗĪŽÓ°Ļģ£»
¢ŚµĪ¶Ø¹ż³ĢÖŠ²»É÷½«ĖįµĪŌŚĘæĶā£¬ĻūŗÄŃĪĖįµÄĢå»żĘ«¶ą£¬²ā¶Ø½į¹ūĘ«“󣬹Ź“š°øĪŖ£ŗĘ«øߣ»
¢ŪµĪ¶ØĒ°ŃöŹÓ”¢µĪ¶Øŗóø©ŹÓ£¬ĻūŗÄŃĪĖįĢå»żĘ«Š”£¬²ā¶Ø½į¹ūĘ«Š”£¬¹Ź“š°øĪŖ£ŗĘ«µĶ£»
¢Ü×°±ź×¼ŅŗÖ®Ē°£¬Ć»ÓŠÓƱź×¼ŅŗČóĻ“µĪ¶Ø¹Ü£¬±ź×¼ŅŗÅØ¶Č¼õŠ”£¬ĻūŗĵÄŃĪĖįŌö¶ą£¬²ā¶Ø½į¹ūĘ«øߣ¬¹Ź“š°øĪŖ£ŗĘ«øߣ®
µćĘĄ ±¾Ģāæ¼²éĮĖµĪ¶Ø²Ł×÷£®²Ł×÷Ź±ŅŖ¹ę·¶£¬·ÖĪöĪó²īŹ±ŅŖæ“ŹĒ·ńÓ°Ļģ±ź×¼Ģå»żµÄÓĆĮ棬Čō±ź×¼Ģå»żĘ«“󣬽į¹ūĘ«øߣ»Čō±ź×¼Ģå»żĘ«Š”£¬Ōņ½į¹ūĘ«Š”£»Čō²»Ó°Ļģ±ź×¼Ģå»ż£¬Ōņ½į¹ūĪŽÓ°Ļģ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| Ę½ŠŠŹµŃ鱹ŗÅ | Na2C2O4ČÜŅŗ £ØmL£© | µĪ¶Ø¹ÜĘšŹ¼¶ĮŹż£ØmL£© | µĪ¶Ø¹ÜµĪ¶ØÖÕµć¶ĮŹż£ØmL£© |
| 1 | 20.00 | 0.00 | 21.18 |
| 2 | 20.00 | 1.02 | 21.00 |
| 3 | 20.00 | 1.18 | 21.20 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
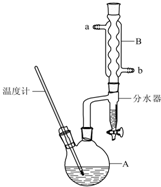 Õż¶”ĆŃ³£ÓĆ×÷ÓŠ»ś·“Ó¦µÄČܼĮ£®ŹµŃéŹŅÖʱøÕż¶”Ćѵķ“Ó¦ŗĶÖ÷ŅŖŹµŃé×°ÖĆČēĻĀ£ŗ
Õż¶”ĆŃ³£ÓĆ×÷ÓŠ»ś·“Ó¦µÄČܼĮ£®ŹµŃéŹŅÖʱøÕż¶”Ćѵķ“Ó¦ŗĶÖ÷ŅŖŹµŃé×°ÖĆČēĻĀ£ŗ| Ļą¶Ō·Ö×ÓÖŹĮæ | ·Šµć/”ę | ĆܶČ/g•cm3 | Ė®ÖŠČܽāŠŌ | |
| Õż¶”“¼ | 74 | 117.2 | 0.8109 | Ī¢ČÜ |
| Õż¶”ĆŃ | 130 | 142.0 | 0.7704 | ¼øŗõ²»ČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| “ż²āŹ³“×µÄĢå»ż³õ¶ĮŹż/mL | 0.02 | 0.03 | 0.00 |
| “ż²āŹ³“×µÄĢå»żÖÕ¶ĮŹż/mL | 25.01 | 25.04 | 25.02 |
| ĒāŃõ»ÆÄʱź×¼ŅŗµÄĢå»ż³õ¶ĮŹż/mL | 0.01 | 0.03 | 0.04 |
| ĒāŃõ»ÆÄʱź×¼ŅŗµÄĢå»żÖÕ¶ĮŹż/mL | 12.52 | 12.55 | 12.58 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 2SO2£Øg£©+O2£Øg£©?2SO3£Øg£©”÷H=-198kJ•mol-1·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
2SO2£Øg£©+O2£Øg£©?2SO3£Øg£©”÷H=-198kJ•mol-1·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
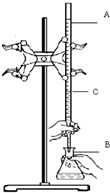 ŌŚŹµŃéŹŅÓĆŅŃÖŖÅØ¶ČµÄŃĪĖįµĪ¶ØijĪ“ÖŖÅØ¶ČµÄNaOHČÜŅŗ£¬×°ÖĆŗĶ²Ł×÷ČēÓŅĶ¼ĖłŹ¾£®Ēė»Ų“š£ŗ
ŌŚŹµŃéŹŅÓĆŅŃÖŖÅØ¶ČµÄŃĪĖįµĪ¶ØijĪ“ÖŖÅØ¶ČµÄNaOHČÜŅŗ£¬×°ÖĆŗĶ²Ł×÷ČēÓŅĶ¼ĖłŹ¾£®Ēė»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Čܽā”¢¹żĀĖ”¢Õō·¢ | B£® | Čܽā”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ | ||
| C£® | Čܽā”¢¹żĀĖ”¢½į¾§ | D£® | Čܽā”¢½į¾§”¢øÉŌļ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com