
 ��ʽ�����������Ƥ�ӡȾ����ҵ��
��ʽ�����������Ƥ�ӡȾ����ҵ������ ��1��ˮԡ���ȿɱ��ֺ㶨���¶ȣ���ֹ�¶ȹ�����ͣ�
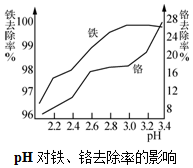
��2�����������ױ������������������ӣ���ͼ��֪pHΪ3.0����ȥ���ʽϴ�
��3�����c��Cr3+����c��OH-��3=Ksp[Cr��OH��3]���㣻
��5����Ϊ��ȡ���������ڲ�ͬ�ܼ��е��ܽ��Բ����йأ�
�����������£����ǻ�ԭ�ظ�����Ҳ���Ʊ�ˮ���Լ�ʽ�������
��1�����ݷ�ӦNa2Cr2O7+NaHSO4+C12H22O11��Cr��OH��SO4+Na2SO4+H2O+CO2����֪��C12H22O11��̼�Ļ��ϼ۴�0�����ߵ�+4�ۣ�Na2Cr2O7�и���+6�۽�Ϊ+3�ۣ����ݵ��ӵ�ʧ�غ�ɼ����1mol C12H22O11�ܻ�ԭNa2Cr2O7�����ʵ�����
��2������Һ�к���Cr��OH��SO4��Na2SO4��������Һ��õ�Cr��OH��SO4��Ʒ�л��е���Ҫ������Na2SO4��
��� �⣺��1�����ȡһ���������������������ᣬˮԡ���ȣ����裬����Ӧ��ȫ����ˣ��ð�ˮ������ҺpH��Լ2.0��ˮԡ���ȵ��ŵ������Ⱦ��ȣ��¶����ڿ��ƣ�
�ʴ�Ϊ�����Ⱦ��ȣ��¶����ڿ��ƣ�
��2���������ȡҺ���������������Ӧ�����ӷ���ʽ��4Fe2++O2+4H+=2Fe3++2H2O����ͼ��֪�������Ӱ�ˮ�������յ�pHΪ3.0��ԭ��������ȥ���ʺܸߣ�������ȥ���ʲ��Ǻܸߣ�
�ʴ�Ϊ��4Fe2++O2+4H+=2Fe3++2H2O������ȥ���ʺܸߣ�������ȥ���ʲ��Ǻܸߣ�
��3��c��Cr3+����10-5 mol/L������Ϊ������ȫ��Ksp[Cr��OH��3]=6.4��10-31����c��Cr3+����c��OH-��3=Ksp[Cr��OH��3]��֪Cr3+��ȫ����ʱ��Һ��c��OH-��Ӧ��С��$\root{3}{\frac{6.4��10{\;}^{-31}}{10{\;}^{-5}}}$=4��10-9mol/L���ʴ�Ϊ��4��10-9��
��5������Ʒ�к�����CrO42-���ⶨ�京��ʱ������TBP�ܼ���ȡ��Һ�е�CrO42-��ѡ��TBP��Ϊ��ȡ����������CrO42-��TBP�е��ܽ�ȴ�������ˮ�е��ܽ�ȣ���TBP������ˮ��
�ʴ�Ϊ��CrO42-��TBP�е��ܽ�ȴ�������ˮ�е��ܽ�ȣ���TBP������ˮ��
�����������£����ǻ�ԭ�ظ�����Ҳ���Ʊ�ˮ���Լ�ʽ�������
��1�����ݷ�ӦNa2Cr2O7+NaHSO4+C12H22O11��Cr��OH��SO4+Na2SO4+H2O+CO2����֪��C12H22O11��̼�Ļ��ϼ۴�0�����ߵ�+4�ۣ�1mol C12H22O11��ʧ48mol���ӣ�Na2Cr2O7�и���+6�۽�Ϊ+3�ۣ�1mol Na2Cr2O7�ܵ�6mol���ӣ����ݵ��ӵ�ʧ�غ��֪��1mol C12H22O11�ܻ�ԭNa2Cr2O7�����ʵ���Ϊ8mo��
�ʴ�Ϊ��8��
��2������Һ�к���Cr��OH��SO4��Na2SO4��������Һ��õ�Cr��OH��SO4��Ʒ�л��е���Ҫ������Na2SO4��
�ʴ�Ϊ��Na2SO4��
���� ���⿼���Ʊ�ʵ�鷽������ƣ�Ϊ�߿����㣬���շ����ķ�Ӧ���ܶȻ����㡢��ȡԭ��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
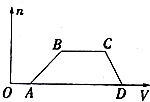 ij�����Һ�п��ܺ���H2SO4��MgCl2��Al2��SO4��3��NH4Cl��NaCl�еļ������ʣ�������Һ������NaOH��Һ���������������ʵ�����n��������NaOH��Һ�����V���Ĺ�ϵ��ͼ��ʾ���ش��������⣺
ij�����Һ�п��ܺ���H2SO4��MgCl2��Al2��SO4��3��NH4Cl��NaCl�еļ������ʣ�������Һ������NaOH��Һ���������������ʵ�����n��������NaOH��Һ�����V���Ĺ�ϵ��ͼ��ʾ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ӵ���һ�������� | |
| B�� | ͨ����˵����Ԫ����ָ${\;}_{1}^{1}$H | |
| C�� | 16O�еġ�16����ʾ��Ԫ�صĽ������ԭ������ | |
| D�� | ���ݷ�ӦK35ClO3+6H37Cl=KCl+3Cl2��+3H2O�õ���Cl2������Է�������Ϊ73.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | M/NA��ʾ�����嵥�����ӵ����� | B�� | mNA/M��ʾ������ķ����� | ||
| C�� | M/22.4��ʾ��������ܶ� | D�� | VM/mNA��ʾ������һ�����ӵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ѩ�ס�Ư���ġ���ľ�������������������Ѭ�ƵĹ����в�����SO2���£�ʳ�ö�����ĸΡ���������������°����� | |
| B�� | �����Թ������������ᷴӦ�Ƶù��ᣬ���ɵĹ����ۺ϶��γɽ�����ҺΪ�轺���轺������ʳƷ������ʹ������� | |
| C�� | ���廯ѧ��Ӧ�úܹ㣬���Ʊ����ײ��ϵ���Ч����֮һ��ij���ϵ�ֱ����1��100nm֮�䣬�ò��Ͼ��ȷ�ɢ��ijҺ���ɢ���У��÷�ɢϵ�ɲ��������ЧӦ | |
| D�� | ��ҽ��������������������������ȱ����ƶѪ��ҩ�����ڹ�ҵ��������������������ϵ�о�ˮ���������������죨��Ҫ�ɷ�ΪFe2O3����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | �״� | ������ | ��������� |
| �е�/�� | 64.7 | 249 | 199.6 |
 C6H5COOCH3+H218O��
C6H5COOCH3+H218O��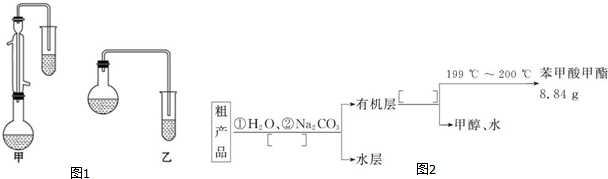
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ԭ��ʧȥ����Խ�࣬��ԭ��Խǿ | |
| B�� | �����ữ�ĸ��������Һ�����Ը�ǿ | |
| C�� | ǿ��������ǿ��ԭ����һ���ܷ���������ԭ��Ӧ | |
| D�� | ��ҵ��þ�����ʶ��ǵ���Ӧ���ڵ��Ȼ���õ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com