
����Ŀ������ѧ����ѡ��3�����ʽṹ�����ʡ�Ŀǰ�뵼������չ����һ����ͭоƬ���������ڹ�оƬ����ͭ���������ߣ����ϵĽ���ͭ���ִ��Ƽ�Ӧ����ȡ����ͻ�ƣ��û�ͭ��(��Ҫ�ɷ�ΪCuFeS2)������ͭ���䷴Ӧԭ�����£�


��1����̬ͭԭ�ӵ���Χ�����Ų�ʽΪ__________________������Ԫ����ȣ���һ�����ܽϴ��Ԫ����________(��Ԫ�ط���)��
��2����Ӧ�������о���������ͬ��������ӣ��÷��ӵ�����ԭ���ӻ�������___��������ṹ��____��
��3��ijѧ��������ͭ��Һ�백ˮ����һ��ʵ�飺CuSO4��Һ![]() ��ɫ����
��ɫ����![]() �����ܽ⣬�õ���
�����ܽ⣬�õ���
��ɫ����Һ��д����ɫ�������ڰ�ˮ�����ӷ���ʽ______������ɫ����Һ�е�������(������H��)�ڴ��ڵ�ȫ����ѧ�������� ��
��4��ͭ�ǵ�����������Ҫ�Ĺ���Ԫ��֮һ���䵥�ʼ���������й㷺��;��ͭ������ͭԭ�Ӷѻ�ģ��Ϊ_____________��ͭ��ij�������ᄃ���ṹ��ͼ��ʾ�����þ�����ܶ�Ϊd g/cm3�������ӵ�������ֵΪNA����þ�����ͭԭ������ԭ��֮��ľ���Ϊ________pm��((�ú�d��NA��ʽ�ӱ�ʾ)��
���𰸡���1��3d104s1 ��2�֣� O ��1�֣� ��2��sp2��2�֣� V�Σ�2�֣�
��3��Cu(OH��2+4NH3��H2O��[Cu(NH3��4]2++2OH-+4H2O ��2�֣� ���ۼ�����λ����2�֣�
��4�������������ܶѻ���1�֣�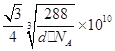 ��3�֣�
��3�֣�
�������������������1�����ݹ���ԭ����֪����̬ͭԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d 104s1�����Ի�̬ͭԭ�ӵ���Χ�����Ų�ʽΪ3d104s1��ͬ����Ԫ�ص�һ���������϶�����С�����Ե�һ�����ܽϴ����O��
��2����Ӧ���������ɵ���������Ƕ�������SO2�м۲���ӶԸ���=2+![]() (6-2��2)=3������Sԭ�Ӳ���sp2�ӻ������ں���һ���µ��Ӷԣ���ռ乹����V�ͣ�
(6-2��2)=3������Sԭ�Ӳ���sp2�ӻ������ں���һ���µ��Ӷԣ���ռ乹����V�ͣ�
��3������ͭ��Һ�백ˮ����������ͭ��ɫ������������ͭ���ڹ����İ�ˮ���γ�[Cu(NH3)4]2+���ӣ���ɫ�������ڰ�ˮ�����ӷ���ʽΪCu(OH)2+4NH3H2O=[Cu(NH3)4]2++2OH-+4H2O������ɫ����Һ�е�������(������H+)�ڴ��ڵ�ȫ����ѧ�������й��ۼ�����λ����
��4��ͭ������ͭԭ�Ӷѻ�ģ��Ϊ�����������ܶѻ�����ͭ��ij�������ᄃ���У�Oԭ���ھ����Ķ�������ģ���Oԭ����=![]() ��8+1=2��Cuԭ��ȫ�������ģ���Cuԭ����=4����һ��������ͭ��������2��Oԭ�Ӻ�4��Cuԭ�ӡ��辧���ı߳�Ϊx�������ܶȿɱ�ʾΪd g/cm3��
��8+1=2��Cuԭ��ȫ�������ģ���Cuԭ����=4����һ��������ͭ��������2��Oԭ�Ӻ�4��Cuԭ�ӡ��辧���ı߳�Ϊx�������ܶȿɱ�ʾΪd g/cm3�� ��
��![]() ��ͭԭ������ԭ��֮��ľ���Ϊ
��ͭԭ������ԭ��֮��ľ���Ϊ![]() x��
x��![]()
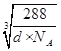 cm��
cm��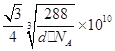 pm��
pm��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ӽ���Ĥ�������ӽ���Ĥ��ʯī�缫����ͼZ99��ʾ���ۡ����ȼҵ�е����ӽ�������ԭ�����ɵ��Na2SO4��Һ����NaOH��H2SO4��Һ��

����˵����ȷ����( )
A��������ӦʽΪ2H����2e��===H2��
B����A�ڳ�������H2SO4��Һ
C��a�������ӽ���Ĥ������H��ͨ��
D��Na2SO4��Һ��E�ڼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����°����ҹ�������ѧ�ң�1933����桶�������졷һ�飬�������й��Լ����Ƽ�ա��䴿������ԭ������ͼ��ʾ��

��1����NaHCO3�Ʊ�����Ļ�ѧ����ʽ��_________________��
��2��������ͨ��NH3��CO2��˳����__________________��
��3������������NH4Cl(s)��ԭ����_____________��
��4��������ø���ƷNH4Cl����NH3��HCl��ֱ�Ӽ��ȷֽ�NH4Cl��NH3��HCl�IJ��������ܵͣ�ԭ����________________��
��5������MgOѭ���ֽ�NH4Cl�����ȣ���300�����»��NH3������������350����600�����HCl���塣��������װ��(���ȼ��ӳ�װ����)�ɲ���NH3��HCl�IJ��ʡ�

�� ����300��ʱ�������ʢװ�ĸ������_________��NH3������ȫ���������������װ���е�����Һ��
�� ����NH3�Ļ�ѧ��Ӧ����ʽ��_________________��
�� ��350����600������ͼ���߿���Ӧѡ��İ�ȫƿ��________��

�� MgO����ѭ���ֽ�NH4Cl�Ƶ�NH3��HCl��ԭ����____________(��ϻ�ѧ����ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ݸ�Ŀʰ�š�����ҩ�������dz�ˮ����Ŀ��д�������Բ���ƿ��������ڣ���ʹй������ҩ���������������ң������ԣ����в��������������ˮ����Ŀ��д�����ν��Դ�ˮͿ�˿ڣ���������ʡ�������˵�����dz�ˮ����������ˮ���ֱ�ָ����
A. ����ᡢʳ��ˮ B. ��ˮ����� C. ʯ��ˮ������ D. ϡ���ᡢʳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2S2O3�׳ƴ��մ���������Ҫ�Ļ���ԭ�ϡ���Na2SO3�������ˮ��Һ�м��ȷ�Ӧ�������Ƶ�Na2S2O3����֪10����70��ʱ��Na2S2O3��100gˮ�е��ܽ�ȷֱ�Ϊ60.0g��212g�������£�����Һ�������ľ�����Na2S2O3��5H2O��
��ʵ��������ȡNa2S2O3��5H2O���壨Na2S2O3��5H2O�ķ�����Ϊ248���������£�
�ٳ�ȡ12.6g Na2SO3���ձ��У�����80.0mLˮ��
����ȡ4.0g��ۣ��������Ҵ���ʪ�ӵ�������Һ�С�
�ۣ���ͼ��ʾ������װ����ȥ����ˮԡ���ȣ��У���ӦԼ1Сʱ����ˡ�
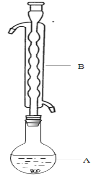
����Һ�ھ��� �� ������Na2S2O3��5H2O���塣
�ݽ��м�ѹ���˲����
��1������B��������________����������___________�������������Ҵ���ʪ��Ŀ���� ��
��2�������Ӧ��ȡ�IJ����� �� ��
��3����Һ�г�Na2S2O3�Ϳ���δ��Ӧ��ȫ��Na2SO3�⣬����ܴ��ڵ��������� �������Һ�и����ʵĺ������ܵͣ�����ķ����ǣ� ��
��4��Ϊ�˲��Ʒ�Ĵ��ȣ���ȡ7.40g ��Ʒ�����Ƴ�250mL��Һ������Һ����ȡ25.00mL����ƿ�У��μ�������Һ��ָʾ��������Ũ��Ϊ0.0500mol/L �ĵ�ˮ���� �����ʽ����ʽ�����ζ������ζ���2S2O32�� + I2 �� S4O62�� + 2I�������ζ�������£�
�ζ����� | �ζ�ǰ������mL�� | �ζ��ζ��������mL�� |
��һ�� | 0.30 | 31.12 |
�ڶ��� | 0.36 | 31.56 |
������ | 1.10 | 31.88 |
�����ò�Ʒ�Ĵ���Ϊ ������ΪӰ�촿�ȵ���Ҫԭ���ǣ������Dz��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ɱ��ۻ�������ʱ�����и����з����仯���ǣ� ��
A.�����ڻ�ѧ�� B.���Ӽ���� C.���ӹ��� D.���Ӽ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��W������X��Z��W����Ԫ�ؿ����XH3��H2Z��HW���ۻ����Y����Ԫ�ؿ����Y2O��Y2O2���ӻ����
��1��д��Y2O2�ĵ���ʽ��_________________�����к��еĻ�ѧ����_______________��
��2���õ���ʽ��ʾY2O���γɹ��̣�____________________________________��
��3����X��W��ɵĻ���������У�X��Wԭ�ӵ��������ﵽ8�����ȶ��ṹ���û�������ˮ������һ�־���Ư���ԵĻ������д����Ӧ�Ļ�ѧ����ʽ��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ�����м׳ص��ܷ�ӦʽΪ2CH3OH+3O2+4KOH=2K2CO3+6H2O������˵����ȷ����������

A. �׳��ǵ���ת��Ϊ��ѧ�ܵ�װ�ã��ҡ������ǻ�ѧ��ת��Ϊ���ܵ�װ��
B. �׳�ͨ��CH3OH�ĵ缫��ӦʽΪCH3OH-6e-+2H2O=CO32-+8H+
C. ��Ӧһ��ʱ������ҳ��м���һ����Cu(OH)2������ʹCuSO4��Һ�ָ���ԭŨ��
D. �׳�������280mL(��������)O2����ʱ������������������1.45g����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ������Ԫ�������ɽ��͵�ֻ�У� ��
A. ���ԣ�KOH��NaOH
B. ���ԭ��������Ar��K
C. ���ԣ�HClO4��H2SO4
D. Ԫ�صĽ����ԣ�Mg��Al
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com