
(6��)
һ���¶��£����ij��ˮ��pHΪ6��5���Լ��㣺
(1)��ʱˮ�����ӻ�����KwΪ����?
(2)���ڴ˴�ˮ�м�һ����Ba(OH)2���壬���Ƴ�0��005mol��L-1����Ba(OH)2��Һ������ԭ�¶ȣ�����Һ��pHΪ����?
(3)ȡ����Ba(OH)2��Һ200mL�����뺬0.01mol![]() ��Ũ��Һ������Ӧ��ȫ���ϲ���Һ��Ba2+Ũ�ȱ�Ϊ����?[����仯���Բ��ƣ�Ksp(BaSO4)=1��08��10-10]
��Ũ��Һ������Ӧ��ȫ���ϲ���Һ��Ba2+Ũ�ȱ�Ϊ����?[����仯���Բ��ƣ�Ksp(BaSO4)=1��08��10-10]
 ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲�ջ�Զ�ذ�����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(6��)һ���¶��£���һ�̶��ݻ����ܱ������г���������������������Ӧ��H2(g)��Br2(g) 2HBr(g)���ﵽƽ��״̬������˵����һ����ȷ���� ����˵���˷�Ӧ�ﵽƽ��״̬���� ��
2HBr(g)���ﵽƽ��״̬������˵����һ����ȷ���� ����˵���˷�Ӧ�ﵽƽ��״̬���� ��
| A����λʱ��������n mol H2��ͬʱ����2n mol HBr |
| B����λʱ��������n mol H2��ͬʱ����n molBr2 |
| C��һ��H��H�����ѵ�ͬʱ������H��Br������ |
| D����HBr�ݡã�H2�ݡã�Br2��=2��2��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09��10ѧ���㽭��ɽ��У�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�������
(6��)
һ���¶��£����ij��ˮ��pHΪ6��5���Լ��㣺
(1)��ʱˮ�����ӻ�����KwΪ����?
(2)���ڴ˴�ˮ�м�һ����Ba(OH)2���壬���Ƴ�0��005mol��L-1����Ba(OH)2��Һ������ԭ�¶ȣ�����Һ��pHΪ����?
(3)ȡ����Ba(OH)2��Һ200mL�����뺬0.01mol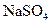 ��Ũ��Һ������Ӧ��ȫ���ϲ���Һ��Ba2+Ũ�ȱ�Ϊ����?[����仯���Բ��ƣ�Ksp(BaSO4)=1��08��10-10]
��Ũ��Һ������Ӧ��ȫ���ϲ���Һ��Ba2+Ũ�ȱ�Ϊ����?[����仯���Բ��ƣ�Ksp(BaSO4)=1��08��10-10]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲�ջ�Զ�ذ�����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
(6��)һ���¶��£���һ�̶��ݻ����ܱ������г���������������������Ӧ��H2(g)��Br2(g) 2HBr(g)���ﵽƽ��״̬������˵����һ����ȷ���� ����˵���˷�Ӧ�ﵽƽ��״̬���� ��
2HBr(g)���ﵽƽ��״̬������˵����һ����ȷ���� ����˵���˷�Ӧ�ﵽƽ��״̬���� ��
A����λʱ��������n mol H2��ͬʱ����2n mol HBr
B����λʱ��������n mol H2��ͬʱ����n molBr2
C��һ��H��H�����ѵ�ͬʱ������H��Br������
D����HBr�ݡã�H2�ݡã�Br2��=2��2��1
E���¶Ⱥ����һ��ʱ��ijһ�������Ũ�Ȳ��ٱ仯
F���¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10ѧ���㽭��ɽ��У�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�������
(6��)
һ���¶��£����ij��ˮ��pHΪ6��5���Լ��㣺
(1)��ʱˮ�����ӻ�����KwΪ����?
(2)���ڴ˴�ˮ�м�һ����Ba(OH)2���壬���Ƴ�0��005mol��L-1����Ba(OH)2��Һ������ԭ�¶ȣ�����Һ��pHΪ����?
(3)ȡ����Ba(OH)2��Һ200mL�����뺬0.01mol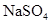 ��Ũ��Һ������Ӧ��ȫ���ϲ���Һ��Ba2+Ũ�ȱ�Ϊ����?[����仯���Բ��ƣ�Ksp(BaSO4)=1��08��10-10]
��Ũ��Һ������Ӧ��ȫ���ϲ���Һ��Ba2+Ũ�ȱ�Ϊ����?[����仯���Բ��ƣ�Ksp(BaSO4)=1��08��10-10]
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com