
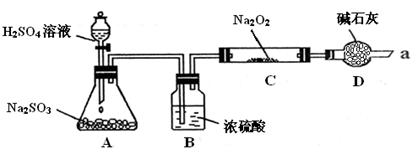

| ʵ�鲽�� | ʵ������ |
| �ٵμ��������ϡ���� | �����ݼ�����ζ���� |
| �ڵμ���������BaCl2��Һ | ������ɫ������ |
| ��ȡ����C�й���������Թ��У���������������ˮ�ܽ� | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
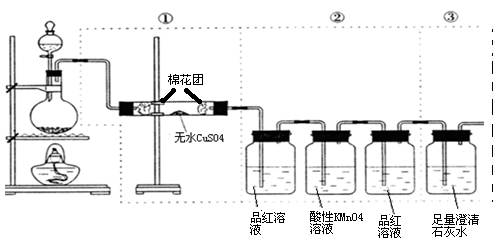
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��Һ����������KMnO4��Һ������Ӧ2MnO4����5H2C2O4��6H��
��Һ����������KMnO4��Һ������Ӧ2MnO4����5H2C2O4��6H��| �ζ����� | ���������Һ���(mL) | 0.1000 mol/LKMnO4����Һ���(mL) | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 10.02 |
| �ڶ��� | 25.00 | 0.22 | 11.32 |
| ������ | 25.00 | 1.56 | 11.54 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
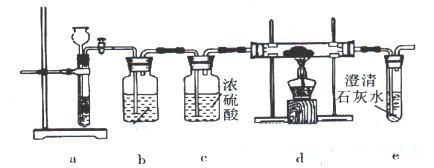
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���� ��˵����Ӧ���ȡ�������װ����֧�ż��������������ȥ��
���� ��˵����Ӧ���ȡ�������װ����֧�ż��������������ȥ��
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ
չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ �ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | - |
 (�����)��
(�����)��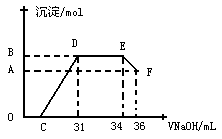
 ��Ӧ�ij��������ʵ���Ϊ________mol��C���Ӧ������������Һ�����Ϊ___________mL
��Ӧ�ij��������ʵ���Ϊ________mol��C���Ӧ������������Һ�����Ϊ___________mL�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
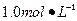 Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��
Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��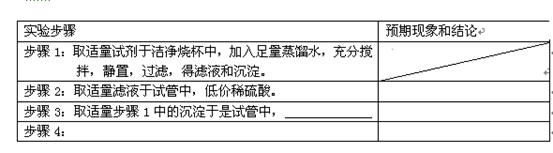
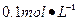 Ba(OH)2��8H2O��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�� ������Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ�
Ba(OH)2��8H2O��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�� ������Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ� ����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��
����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��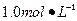 Ba(OH)2��Һ��
Ba(OH)2��Һ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com