
 ijѧ����0.1000 mol•L-1KOHҺ�ζ�δ֪Ũ�ȵĴ��ᣬ������ֽ�Ϊ���¼�����
ijѧ����0.1000 mol•L-1KOHҺ�ζ�δ֪Ũ�ȵĴ��ᣬ������ֽ�Ϊ���¼�����| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�������mL�� | 20.05 | 20.00 | 18.80 | 19.95 |
���� ��1�������к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ��Ȳ�����
��2�����ݼ�ˮ����ҺŨ�ȵ�Ӱ�������
��3������c�����⣩=$\frac{c��������V������}{V�����⣩}$�жϲ��������������������Ӱ�죻
��4���������������ط�Ӧ���ɵĴ�����Լ��ԣ�����Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
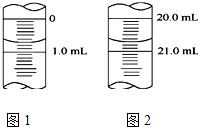
��5���ζ��ܵĿ̶����϶��¿̶�������Ϊ0.01mL���ݴ˽��ͼ�������
��6�����ݹ�ϵʽHCl��NaOH������������Ũ�ȣ�
��� �⣺��1���к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ���˳�����������ȷ��˳��Ϊ��B��D��C��E��A��F��
�ʴ�Ϊ��B��D��C��E��A��F��
��2���ζ���������ˮϴ���ḽ������������ˮ����ʹ����Һ��Ũ�ȼ�С������Ҫ�ñ���Һ��ϴ�ζ���2��3�Σ�
�ʴ�Ϊ����ֹ�ζ����ڱڵ�ˮ����Һϡ�ͣ�
��3����ƿ������ˮϴ�Ӻ�������ô���Һ��ϴ����ʹ��ƿ�����ʵ����ʵ����������V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$������֪�������c�����⣩ƫ��
�ʴ�Ϊ��ƫ��
��4���������������ط�Ӧ���ɵĴ�����Լ��ԣ��÷�̪��ָʾ���������յ�ʱ�����������һ����Һʱ����ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ��
�ʴ�Ϊ����̪��Һ�����������һ����Һʱ����ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ��
��5���ζ��ܵĿ̶����϶��¿̶�������Ϊ0.01mL����ͼ��֪�ζ�ǰ����Ϊ0.70mL���յ����Ϊ20.70mL���εζ����̹�ʹ����20.00mLKOH��Һ��
�ʴ�Ϊ��0.70��20.00��
��6��������ʵ�����ݲ��ϴ����������ı�NaOH��Һ�����ƽ��ֵΪ20.00mL��
CH3COOH��NaOH
1 1
C��CH3COOH����20.00mL 0.1000mol•L-1��20.00mL��
C��CH3COOH��=0.1000mol•L-1��
�ʴ�Ϊ��0.1000mol•L-1��
���� ���⿼��������к͵ζ��еIJ���Ҫ�㡢�еζ��ļ����Լ�����������Ŀ�ѶȲ�����c�����⣩=$\frac{c��������V������}{V�����⣩}$�����������ڿ���ѧ���ķ��������ͼ���������


 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | �����Լ� | ���ӷ���ʽ |
| A | NH4+��Mg2+��SO42- | ����Ba��OH��2��Һ | NH4++SO42-+Ba2++OH-=BaSO4��+NH3•H2O |
| B | Mg2+��HCO3-��Cl- | ����NaOH��Һ | Mg2++2HCO3-+2OH-=MgCO3-��+CO32-+2H2O |
| C | H+��Na+��NO3- | Fe�� | Fe+2H+=Fe2++H2�� |
| D | Ca2+��NH3•H2O��Cl- | ͨ�����CO2 | NH3•H2O+CO2=NH4++HCO3- |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 25��ʱ��FeS��ZnS��CuS���ܽ���������� | |
| B�� | ZnS������Һ�м�������NazS���壬ƽ�����Һ��c��Zn2+��•c��S2-��=Ksp��ZnS����c��Zn2+��=c��S2-�� | |
| C�� | ��ȥ��ҵ��ˮ�е�Cu2+������FeS��Ϊ������ | |
| D�� | ij��Һ�к���Fe2+��Cu2+��Znz+��Ũ�Ⱦ�Ϊ0.010 moI•L-1�������Һ����μ���0.010 mol•L-1��Na2S��Һʱ��Fe2+���ȳ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | Cu��OH��2 | Fe��OH��3 | CuCl | CuI |
| Ksp | 2.2��10-20 | 2.6��10-39 | 1.7��10-7 | 1.3��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
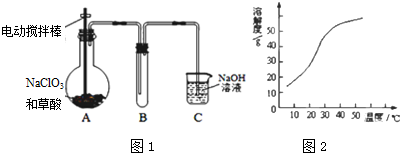
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
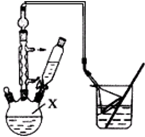 ���嶡�����ӣ�
���嶡�����ӣ�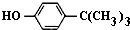 ����ҵ��;�㷺�����������������Է�ȩ��֬���ȶ��������ϵȣ�ʵ�����Ա��ӡ��嶡����[��CH3��3CCl]��Ϊԭ���Ʊ����嶡�����ӣ�
����ҵ��;�㷺�����������������Է�ȩ��֬���ȶ��������ϵȣ�ʵ�����Ա��ӡ��嶡����[��CH3��3CCl]��Ϊԭ���Ʊ����嶡�����ӣ� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
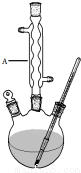 ˮ�����������һ����Ҫ���л��ϳ�ԭ�ϣ�ij��ѧС����ˮ���ᣨ�ṹ��ʽΪ
ˮ�����������һ����Ҫ���л��ϳ�ԭ�ϣ�ij��ѧС����ˮ���ᣨ�ṹ��ʽΪ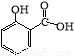 ���ͼ״������Դ������ºϳ�ˮ�����������������ʣ�
���ͼ״������Դ������ºϳ�ˮ�����������������ʣ�| ���� | ������ | ��ɫ״̬ | ����ܶ� | �۵㣨�棩 | �е㣨�棩 |
| ˮ������� | 152 | ��ɫҺ�� | 1.18 | -8.6 | 224 |
| ˮ���� | 138 | ��ɫ���� | 1.44 | 158 | 210 |
| �״� | 32 | ��ɫҺ�� | 0.792 | -97 | 64.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V������Һ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.95 | 15.20 | 15.15 | 16.95 |
| V��NaOH��/mL�����ģ� | 14.95 | 15.00 | 15.05 | 16.95 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com