
�±�ΪԪ�����ڱ���һ���֡���ش��������⣺
����Ԫ�������ڱ��е����λ�ÿ�֪�١���ֱ���H��C��N��O��Mg��Al��Cl��Ca��Mn��Fe��
��1������Ԫ���У�����s������____________(��Ԫ�ط���)��
��2��д��Ԫ�آܵĻ�̬ԭ�ӵļ۵����Ų�ͼ____________________��
��3��Ԫ�ص�һ������Ϊ��________�� (����ڡ���С�ڡ�)��
��4��Ԫ�آ���̬�⻯���VSEPRģ��Ϊ________���÷���Ϊ________����(����ԡ��Ǽ��ԡ�)��������ͭ��Һ����μ�����ˮ��Һ���ɹ۲쵽������Ϊ_____________________________��
��5�����ʵľ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��
����֪��ԭ�Ӱ뾶Ϊdcm��NA���������ӵ�������Ԫ�آ����ԭ������ΪM����ش𣺾����Т�ԭ�ӵ���λ��Ϊ ,�þ�����ܶ�Ϊ ������ĸ��ʾ��
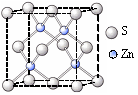
��6��ʵ��֤�����ݺ͢�������KCl��TiN��4�־���Ľṹ��NaCl����ṹ���ƣ�����ͼ��ʾ������֪3�����Ӿ���ľ������������±���
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |
��1��H Mg Ca��2��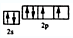 ��
�� ��3������
��3������
��4�������� ���� �Ȳ�����ɫ���������ܽ������ɫ��Һ
��5��12 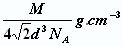 ��6��TiN��MgO��CaO��KCl 6
��6��TiN��MgO��CaO��KCl 6
�����������������Ԫ�������ڱ��е����λ�ÿ�֪�١���ֱ���H��C��N��O��Mg��Al��Cl��Ca��Mn��Fe��
��1���������������ڰ��չ���ԭ�����������ӵĹ�����ƣ����Ը���Ԫ�صĺ�������Ų���֪������Ԫ���У�����s������H��Mg��Ca��
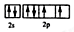
��2����Ԫ�ص�������������6�������Ի�̬ԭ�ӵļ۵����Ų�ͼ�� ��
�� ��
��
��3������MnԪ�ص�3d�ܼ���5�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܴ�������Ԫ�صģ�����MnԪ�صĵ�һ�����ܴ�����Ԫ�صĵ�һ�����ܡ�
��4�����ݼ۲���ӶԻ������ۿ�֪�����������е�Ԫ�غ��еŶԵ��Ӷ�������5��3��1����2��1����������VSEPRģ��Ϊ�����壻��ʵ�ʵĿռ乹���������Σ����Ը÷���Ϊ���Է��ӡ�����ͭ�����ܺͰ����γ���λ��������������ͭ��Һ����μ�����ˮ��Һ���ɹ۲쵽������Ϊ�Ȳ�����ɫ���������ܽ������ɫ��Һ��
��5�����ݽ������ľ�����֪���þ�������λ���ǣ�3��8����2��12��������������ԭ�ӵĸ����� �����ݱ��Ľṹ��֪���þ����ı߳���
�����ݱ��Ľṹ��֪���þ����ı߳���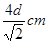 ��������
��������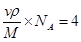 ,��˾������ܶ�
,��˾������ܶ�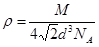 g/cm3��
g/cm3��
��6�����Ӿ�������Ӱ뾶ԽС���������Խ�࣬������Խ��������۷е�Խ�ߣ�����TiN��MgO��MgO��CaO���ɱ������ݿ�֪CaO��KCl������۵�Ӹߵ��͵�˳����TiN��MgO��CaO��KCl���Ȼ��Ƶ���λ����6���������¡����Һ�ǰ���1��������Ca�������ᄃ����һ����������Χ�������ڽ��ҵȾ������������6����
���㣺����Ԫ�����ڱ��Ľṹ���۵����Ų�ͼ����λ������һ�����ܡ����ӵĿռ乹���Լ��������͵��йؼ��㡢�жϺ��۵�Ƚϵ�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ��������������������������Ҫ��Ԫ�ء�λ�������ԡ����߹�ϵ���ۺϿ��飬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ��������������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������������ѵ��Ǿ����ܶȵļ��㡣


 ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±���Ԫ�����ڱ���һ���֣�����Ҫ��ش��������⡣
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 2 | | | | E | H | F | I | |
| 3 | A | C | D | | | | G | R |
| 4 | B | | | | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Aԭ�ӵ����������Ų�ʽΪnsnnpn+1��C��Dԭ�Ӻ��ⶼ��һ��δ�ɶԵ��ӣ���֪B��EΪͬһ���壬C��D��EΪͬ���ڣ���B��E��ԭ������֮�͵���C��D��ԭ������֮��
(1) E���ӵĻ�̬�����Ų�ʽ _______________
(2)Ԫ��B��C��D��E��ԭ�Ӱ뾶�Ӵ�С��˳����________(��Ԫ�ط�����д,��ͬ)
(3)Ԫ��A��B��C��D�ĵ�һ�����ܴӴ�С��˳����
(4)д��C������B���ʼ���ʱ���ɵĻ�����ĵ���ʽ
(5) DԪ�������ڱ��е�λ����_______________����ѧ��ѧʵ��������ȡD������������Ӧ��ˮ��������ӷ���ʽΪ___________________________________
(6)��֪����A2(g)+B2(g)=2AB(g) ��H1=akJ/mol
��2AB(g)+B2(g)=2AB2(g) ��H2=bkJ/mol
��AB(g)+AB2(g)= A2B3(g) ��H3=ckJ/mol
��2A2(g)+3B2(g)=2A2B3(g) ��H= kJ/mol (��a.b.c��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ֶ���������Ԫ��A��B��C�� D��E����ԭ��������������Aԭ��Լռ������ԭ��������88.6%��A+�ֳ�Ϊ���ӣ�B���γɻ�������������Ԫ�أ�CԪ�ص�����⻯��Y��ˮ��Һ�Լ��ԣ�E�Ƕ�����Ԫ���е縺����С��Ԫ�ء�A��B��C��E����Ԫ�ض�����DԪ���γ�ԭ�Ӹ����Ȳ���ͬ�ij���������Իش��������⣺
��1��д��A��E��Ԫ���γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ�����ĵ���ʽ____��
��2�����Ȼ�������Һ�μӹ�����E������������Ӧˮ�������Һ��������____��
��3��Y��Һ�Լ��Ե�ԭ����(��һ�����ӷ���ʽ��ʾ��____��
��4����������β���к��еĻ�����BD�ķ����ǣ�������PdC12��Һ��ͨA����β���������ɺ�ɫ������Pd����֤������β���к���BD��д����Ӧ�����ӷ���ʽ____��
��5�������й��������ʵıȽ��У�����ȷ���� ��
a�����ȶ��ԣ�H2S>SiH4 b�����Ӱ뾶��Na+>S2��
c����һ������N>O d��Ԫ�ص縺�ԣ�C>H
��6����֪����CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H=+49.0kJ/mol
��CH3OH(g)+3/2O2(g)=CO2(g)+2H2O(g) ��H=��192��9kJ/mol
����������ʽ��֪��CH3OH��ȼ����____������ڡ��������ڡ���С�ڡ���192.9kJ/mol����֪ˮ��������Ϊ44 kJ/mol�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬��̬��ԭ��M���ϵ�δ�ɶԵ�����Ϊ ��
��2�������ѧ�����������л�̫���ܹ�����Ч��ͻ��5.3�������ߴ���C60���䡰������������C60�Ľṹ��ͼ1��������̼ԭ�ӹ�����ӻ�����Ϊ ��1 mol C60�����Цм�����ĿΪ ��
��3������̪ݼ������ڹ�̫���ܵ��������Ҫ���ã�һ�ֽ���þ̪ݼ�����Ľṹ����ͼ2���ýṹ�У�̼��֮��Ĺ��ۼ������� ����ԭ�ӹ���ص���ʽ��д���ۼ������ͣ���������ͼ2���ü�ͷ��ʾ����λ����

ͼ1 ͼ2 ͼ3
��4����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ���п��ͭ������Ĥ��صȡ�
�ٵ�һ�����ܣ�As Se�����������������������
����п�ľ����У��ṹ��ͼ��ʾ���������ӵ���λ���� ��
�۶����������ӵĿռ乹��Ϊ ��
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ӧ�ķ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
W��X��Y��ZΪ���ֶ���������Ԫ�أ���λ�ù�ϵ��ͼ��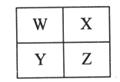
��1����Xԭ�ӵ������������Ǵ�����3����
��Ԫ��X�����ڱ��е�λ��___________________________________��
�ڹ�ҵ����W�ĵ����Ʊ�W����̬�⻯��Ļ�ѧ����ʽ��
_______________________________________________________________________��
��2��������Ԫ����ֻ��һ�ֽ���Ԫ�ء�
��ұ��Y�Ļ�ѧ����ʽ��__________________________________________��
�ڹ�ҵ�ϣ���X�ĵ�����Z�������������ȵ�1900�����Ͽ��Ƶ�һ�������մɣ�ZX����ͬʱ�õ�һ�ֿ�ȼ�����壬�÷�Ӧ�Ļ�ѧ����ʽ��
_________________________________
��Ϊ�Ƚ�X��Z����������ˮ��������ǿ����ijͬѧ�������ʵ�顣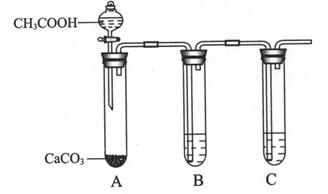
I. B�Թ���ʢ�ŵ��Լ���______________________��
II. C�Թ��з�Ӧ�Ļ�ѧ����ʽ��___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��B��C�ǵڶ����ڵķǽ���Ԫ�أ���ԭ��������������������֮������γ�AC��BC�Լ�AC2��BC2���ӣ�DԪ����һ�ֶ�����Ԫ�أ�����A��B��C�ɷֱ��γɵ���������ȵ����ַ��ӡ�����գ�
(1)д��A��B��C��D��Ӧ��Ԫ�ط��ţ�A________��B________��C________��D________��
(2)д����ҵ������BD3��������Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(3)����ΪB��C��DԪ���γɵĻ�����֮��________(��ܡ����ܡ�)��������ķ�ӦBC��BD3�D��DBC2��D2C���ó�������۵�������__________________________ _��
(4)��.���º�ѹ�£���һ����ɱ���ܱ������з������з�Ӧ��4AC(g)��2BC2(g)  4AC2(g)��B2(g)�����������£��������г���AC��BC2��1 mol��ƽ��ʱ������AC2��B2��a mol����AC��ת������________(�ú�a�Ĵ���ʽ��ʾ)��
4AC2(g)��B2(g)�����������£��������г���AC��BC2��1 mol��ƽ��ʱ������AC2��B2��a mol����AC��ת������________(�ú�a�Ĵ���ʽ��ʾ)��
��. ��ά���¶Ȳ��䣬��һ�����Ӧǰ����ʼ�����ͬ���ݻ��̶����ܱ������з������������Ļ�ѧ��Ӧ����ʼʱ���������г���AC��BC2��1 mol����ƽ��ʱ����AC2��B2��b mol����b����е�a���бȽϣ���a________b(���������������������ȷ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣�����X��Y��Z��W��R����Ԫ�أ�����ǰ����Ϊ����������Ԫ�ء�Xԭ�ӵ������������Ǻ�����Ӳ�����3����Y���������������۵Ĵ�����Ϊ6��X��Zͬ���壬W��X��Y����ͬ���ڣ�R2+�ļ۵����Ų�ʽΪ3d5��
��1��RԪ�������ڱ��е�λ�� ��W2X2�ĵ���ʽ ��
��2��X��Y��Z����ͼ��⻯���У��е���ߵ��� ���ѧʽ�������ۼ�������С���� ��X��Y��Z���ӻ����� ������ͬ����ͬ����
��3����R�ľ�����ÿ������ƽ������2��Rԭ�ӣ�������ͼ��������?���δ������Rԭ�ӡ�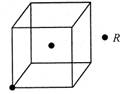
��4����1.19g ZXY2����100mlˮ�з�����������ԭ��Ӧ������2���ᣬ��Ӧ����ʽΪ ��������Һ��������Ũ���ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA<C<B<D<E����֪Aԭ�ӵ�p���Ϊ����������γɵļ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵġ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B�����ӱ�Dԭ���γɵ�������һ�����Ӳ㡣C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29��
��ش��������⣺
(1) Ԫ��A���⻯����Aԭ�ӵ��ӻ�������________��B��C��D�ĵ縺����С�����˳��Ϊ______(������Ӧ��Ԫ�ط��ű�ʾ)��C����̬�⻯��������ˮ��ԭ����____________________��
(2)Eԭ�ӵĻ�̬�����Ų�ʽΪ________��Ԫ��E�ĵ��ʾ����ڲ�ͬ�¶��¿������ֶѻ���ʽ�������ֱ���ͼa��b��ʾ���������������ѻ��ľ��������������ѻ��ľ�����ʵ�ʺ��е�Eԭ�ӵĸ���֮��Ϊ____________��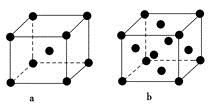
(3)ʵ��֤����KCl��MgO��CaO��TiN��4�־���Ľṹ��NaCl����ṹ����(��ͼ��ʾ)������3�����Ӿ���ľ������������±���
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com