
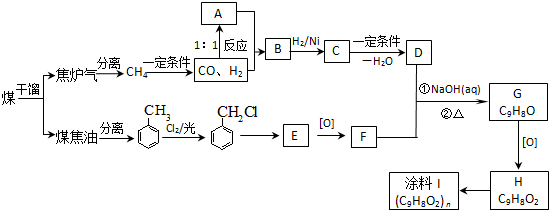
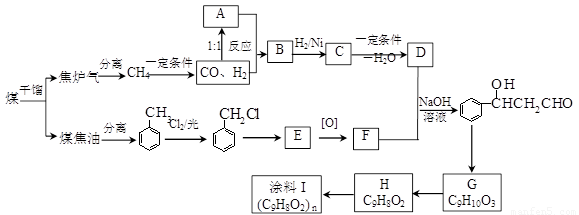
��úΪԭ�Ͽɺϳ���Ҫ�����м���G������B�����ụΪͬ���칹�壬Ҳ�����Ʒ�Ӧ�ų�������ע������ͼʾ������ijЩ��Ӧ������һ����������
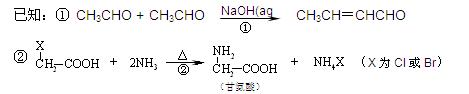

����ա�
��1��C�Ľṹ��ʽ�ǣ� ��
��2��B��C������Ӧ�Ļ��������ǣ� H�����ں������������� ��
��3��Ϳ��I�������ǣ� ��
��4��F + D �� G�Ļ�ѧ����ʽΪ�� ��
��5������Na2CO3��Һ��Ӧ��E�ķ����廯�����ͬ���칹���� �֡�
��6��д��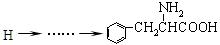 H�ĺϳ�·�߲�ע����Ӧ������
H�ĺϳ�·�߲�ע����Ӧ������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| NaOH(aq) |
| �� |
| �� |
| �� |
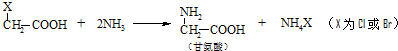

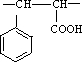

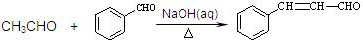
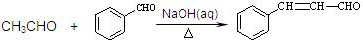
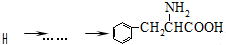 �ĺϳ�·�߲�ע����Ӧ������
�ĺϳ�·�߲�ע����Ӧ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
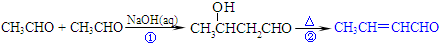
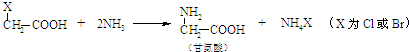
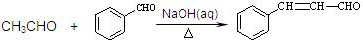
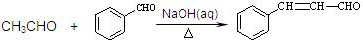
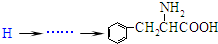 �ĺϳ�·�߲�ע����Ӧ������
�ĺϳ�·�߲�ע����Ӧ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�Ͱ���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12�֣�ú���ۺ������ǵ�����ѧ��ҵ�����ƣ���úΪԭ�Ͽɺϳ���Ҫ�����м���G������B�����ụΪͬ���� ���壬����Na��Ӧ�ų�������
���壬����Na��Ӧ�ų�������
��֪���� CH3CHO + CH3CHO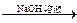
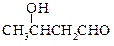
��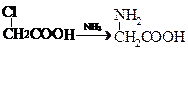 ���ʰ��ᣩ
���ʰ��ᣩ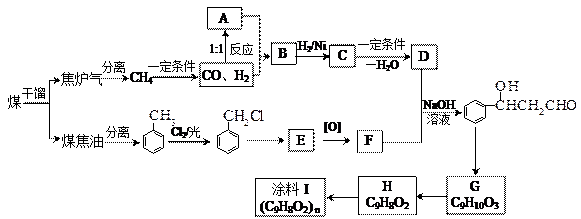
�ش��������⣺
��1��ú�ĸ�������____________�仯��ʵ���Ҽ���D���õ��Լ�Ϊ________________________��
��2��A��F�Ľṹ��ʽ�ֱ�Ϊ_____________________��______________________________��
��3��д��B��C�Ļ�ѧ����ʽ___________________________________________________��
H��I�Ļ�ѧ����ʽ___________________________________________________��
��4������Na2CO3��Һ��Ӧ��E�ķ����廯�����ͬ���칹����________________�֡�
��5��������֪��Ϣ����һ����������G�ϳ�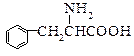 ������Ҫ_______����ѧ��Ӧ��
������Ҫ_______����ѧ��Ӧ��
a��3 b��4 c��5 d��6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�����и�����ѧ��ѧ����飨������ѧ�Ծ� ���ͣ������
��úΪԭ�Ͽɺϳ���Ҫ�����м���G������B�����ụΪͬ���칹�壬Ҳ�����Ʒ�Ӧ�ų�������ע������ͼʾ������ijЩ��Ӧ������һ����������
����ա�
��1��C�Ľṹ��ʽ�ǣ� ��
��2��B��C������Ӧ�Ļ��������ǣ� H�����ں������������� ��
��3��Ϳ��I�������ǣ� ��
��4��F + D �� G�Ļ�ѧ����ʽΪ�� ��
��5������Na2CO3��Һ��Ӧ��E�ķ����廯�����ͬ���칹���� �֡�
��6��д�� H�ĺϳ�·�߲�ע����Ӧ������
H�ĺϳ�·�߲�ע����Ӧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12�֣�ú���ۺ������ǵ�����ѧ��ҵ�����ƣ���úΪԭ�Ͽɺϳ���Ҫ�����м���G������B�����ụΪͬ���칹�壬����Na��Ӧ�ų�������
��֪���� CH3CHO + CH3CHO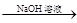
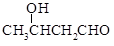
�ش��������⣺
��1��ú�ĸ�������____________�仯��ʵ���Ҽ���D���õ��Լ�Ϊ________________________��
��2��A��F�Ľṹ��ʽ�ֱ�Ϊ_____________________��______________________________��
��3��д��B��C�Ļ�ѧ����ʽ___________________________________________________��
H��I�Ļ�ѧ����ʽ___________________________________________________��
��4������Na2CO3��Һ��Ӧ��E�ķ����廯�����ͬ���칹����________________�֡�
��5��������֪��Ϣ����һ����������G�ϳ� 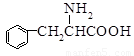 ������Ҫ_______����ѧ��Ӧ��
������Ҫ_______����ѧ��Ӧ��
a��3 b��4 c��5 d��6
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com