
| ķĄĀėÖŹĮæ/g | 50 | 20 | 20 | 10 | 5 |
| ³ĘĮæ£ØČ”ÓĆķĄĀė¹ż³Ģ£© |
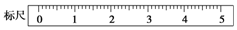
·ÖĪö £Ø1£©ŅĄ¾Żm=CVM¼ĘĖćČÜÖŹµÄÖŹĮ棻
£Ø2£©ĶŠÅĢĢģĘ½µÄ¾«Č·¶ČĪŖ0.1g£¬·Ö¶ČÅĢµÄÖøÕėĘ«ÓŅ£¬ĖµĆ÷ÓŅ±ßÖŲ£¬½«×ó±ßµÄŗįĮŗĀŻĖæĶłĶāµ÷µ÷½ŚĘ½ŗā£»øł¾ŻŹ¹ÓĆĶŠÅĢĢģĘ½Ź±£¬¼ÓķĄĀėµÄÕżČ·²Ł×÷Ķź³É£»
£Ø3£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½ÖčĪŖ£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČ£¬¾Ż“ĖÅÅŠņ£»
£Ø4£©·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæ»ņ¶ŌČÜŅŗµÄĢå»żµÄÓ°Ļģ£¬øł¾Żc=$\frac{n}{V}$·ÖĪöÅŠ¶Ļ£®
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘ500mL 0.5mol/LµÄNaOHČÜŅŗ£¬ŠčŅŖČÜÖŹµÄÖŹĮæm=0.5mol/L”Į0.5L”Į40g/mol=10.0g£¬
¹Ź“š°øĪŖ£ŗ10.0g£»
£Ø2£©·Ö¶ČÅĢµÄÖøÕėĘ«ÓŅ£¬ĖµĆ÷ÓŅ±ßÖŲ£¬×óÅĢøßÓŚÓŅÅĢ£®Ó¦½«×ó±ßµÄŗįĮŗĀŻĖæĶłĶāµ÷ŅŌµ÷½ŚĘ½ŗā£®ĶŠÅĢĢģĘ½µÄ¾«Č·¶ČĪŖ0.1g£¬¹ŹÓ¦ĪŖ32.6g£»øł¾ŻĻČ¼ÓÖŹĮæ“óµÄķĄĀė£¬ŌŁ¼ÓŠ”µÄķĄĀėŌŌņ£¬ĻČŃ”Ōń50gķĄĀė£¬ÖŹĮæĘ«“ó£¬ŌŁ»»20gķĄĀė£¬ķĄĀėÖŹĮæ²»×ć£¬ŌŁ¼Ó20gķĄĀė£¬ķĄĀėÖŹĮæĘ«“ó£¬ŌŁÓĆ10gķĄĀė£¬ķĄĀėÖŹĮæ²»×ć£¬Ōö¼Ó5gķĄĀė£¬ķĄĀėÖŹĮæĘ«“ó£¬ŌŁµ÷½ŚÓŅĀėµ½2.2g£¬
¹Ź“š°øĪŖ£ŗøßÓŚ£»½«×ó±ßµÄĘ½ŗāĀŻÄø×óŠżŅĘ¶Æ£¬»ņ½«ÓŅ±ßµÄĘ½ŗāĀŻÄø×óŠż£¬Ö±ÖĮĢģĘ½Ę½ŗā£»32.6g£» £»
£»
£Ø3£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½ÖčĪŖ£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČ£¬ĖłŅŌÕżČ·µÄ²Ł×÷Ė³ŠņĪŖ£ŗ¢Ł¢Ū¢Ü¢Ś¢Ż£»
¹Ź“š°øĪŖ£ŗ¢Ł¢Ū¢Ü¢Ś¢Ż£»
£Ø4£©Źµ¼ŹÅäÖĘNaOHČÜŅŗµÄÅضČ0.48mol•L-1£¬ĖłÅäČÜŅŗÅضČĘ«µĶ£®
A£® ÓĆĀĖÖ½³ĘĮæŹ±£¬ĀĖÖ½»įĪüø½ĒāŃõ»ÆÄĘ£¬ĖłŅŌ×ŖČėÉÕ±µÄĒāŃõ»ÆÄĘŅ²ÉŁĮĖ£¬ĖłÅäČÜŅŗÅضČĘ«µĶ£®
B£® ×īŗóŠčŅŖ¶ØČŻ£¬ČŻĮæĘæ²»øÉŌļ£¬ŗ¬ÓŠÉŁĮæÕōĮóĖ®£¬¶ŌČÜŅŗÅضČĪŽÓ°Ļģ£»
C£® Ī“Ļ“µÓŅŗÉÕ±”¢²£Į§°ō£¬ŅĘČėČŻĮæĘæÖŠĒāŃõ»ÆÄʵďµ¼ŹÖŹĮæ¼õŠ”£¬ČÜŅŗÅضČĘ«µĶ£»
D£®¶ØČŻŹ±ŃöŹÓæĢ¶Č£¬Ź¹ČÜŅŗµÄĢå»żĘ«“ó£¬ĖłÅäČÜŅŗÅضČĘ«µĶ£»
¹ŹŃ”£ŗACD£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ£¬×¢Ņā“Óc=$\frac{n}{V}$Ąķ½āÅäÖĘŌĄķ£¬×¢ŅāøÆŹ“ŠŌŅ׳±½āµÄŅ©Ę·Ó¦·ÅŌŚ²£Į§Ę÷ĆóÄŚ³ĘĮ森



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
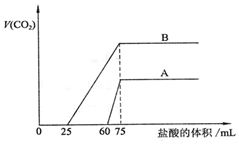 Č”µČĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗĮ½·ŻAŗĶB£¬Ćæ·Ż50mL£¬ĻņĘäÖŠø÷ĶØČėŅ»¶ØĮæµÄCO2£¬Č»ŗóø÷Č”ČÜŅŗ10mL£¬·Ö±š½«ĘäĻ”ŹĶĪŖ100mL£¬ŌŁ·Ö±šĻņĻ”ŹĶŗóµÄČÜŅŗÖŠÖšµĪ¼ÓČė0.1mol/LµÄŃĪĖį£¬±ź×¼×“æöĻĀ²śÉśµÄCO2ĘųĢåĢå»żÓėĖł¼ÓŃĪĖįĢå»żÖ®¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
Č”µČĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗĮ½·ŻAŗĶB£¬Ćæ·Ż50mL£¬ĻņĘäÖŠø÷ĶØČėŅ»¶ØĮæµÄCO2£¬Č»ŗóø÷Č”ČÜŅŗ10mL£¬·Ö±š½«ĘäĻ”ŹĶĪŖ100mL£¬ŌŁ·Ö±šĻņĻ”ŹĶŗóµÄČÜŅŗÖŠÖšµĪ¼ÓČė0.1mol/LµÄŃĪĖį£¬±ź×¼×“æöĻĀ²śÉśµÄCO2ĘųĢåĢå»żÓėĖł¼ÓŃĪĖįĢå»żÖ®¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1.2mol | B£® | 2mol | C£® | 0.8mol | D£® | 1.8mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | Ķ¼ŅŅÖŠY±ķŹ¾¢ņAŌŖĖŲµÄ¼Ūµē×ÓŹż | |
| B£® | Ķ¼¶”ÖŠY±ķŹ¾¢÷AŌŖĖŲĒā»ÆĪļµÄ·Šµć | |
| C£® | Ķ¼±ūÖŠY±ķŹ¾µŚČżÖÜĘŚŌŖĖŲµÄ×īøßÕż»ÆŗĻ¼Ū | |
| D£® | Ķ¼¼×ÖŠY±ķŹ¾F-”¢Na+”¢Mg2+”¢Al3+ĖÄÖÖĄė×ӵİė¾¶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃéŹĀŹµ | ½įĀŪ |
| A | ĘäĖūĢõ¼žĻąĶ¬£¬Na2S2O3ČÜŅŗÅضČŌ½“ó£¬ÓėĶ¬ÅØ¶ČµÄĮņĖį·“Ó¦Īö³öĮ÷³ĮµķĖłŠčµÄŹ±¼äŌ½¶Ģ | µ±ĘäĖūĢõ¼ž²»±äŹ±£¬Ōö“ó·“Ó¦ĪļÅØ¶Č£¬»Æѧ·“Ó¦ĖŁĀŹŌö“ó |
| B | ÓĆĶ×÷µē¼«µē½āCuSO4ČÜŅŗ£¬CuSO4ČÜŅŗÅØ¶Č²»±ä | Cu2+ƻӊ²ĪÓėµē¼«·“Ó¦ |
| C | ŌŚ³£ĪĀĻĀN2²»ÄÜÓėO2·“Ó¦£¬¶ų°×Į×Č“ÄÜ×ŌČ¼ | µŖŌŖĖŲµÄ·Ē½šŹōŠŌ±ČĮ×ŌŖĖŲµÄČõ |
| D | “×ĖįÄÜÓė¼ī·“Ó¦ | “×Ėį·Ö×ÓŅ»¶Ø²»ÄÜ“ęŌŚÓŚ¼īŠŌČÜŅŗÖŠ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| X | Y | ||
| Z | W |
| A£® | ŌŖĖŲµÄ×īøß»ÆŗĻ¼ŪZ“óÓŚY | |
| B£® | ŌŖĖŲµÄĘųĢ¬Ēā»ÆĪļµÄĪČ¶ØŠŌWĒæÓŚY | |
| C£® | Ō×Ó°ė¾¶Z±ČYŠ” | |
| D£® | ŌŖĖŲµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļĖįŠŌX“óÓŚW |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com