
ÅŠ¶ĻÕżĪó£¬ÕżČ·µÄ»®”°”Ģ”±£¬“ķĪóµÄ»®”°”Į”±
(1)S(s)£«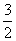 O2(g)===SO3(g)””¦¤H£½£315 kJ·mol£1(Č¼ÉÕČČ)””(¦¤HµÄŹżÖµÕżČ·)(””””)
O2(g)===SO3(g)””¦¤H£½£315 kJ·mol£1(Č¼ÉÕČČ)””(¦¤HµÄŹżÖµÕżČ·)(””””)
(2)NaOH(aq)£«HCl(aq)===NaCl(aq)£«H2O(l)
¦¤H£½£57.3 kJ·mol£1(ÖŠŗĶČČ)””(¦¤HµÄŹżÖµÕżČ·)(””””)
(3)ŅŃÖŖH£«(aq)£«OH£(aq)===H2O(l)””¦¤H£½£57.3 kJ·mol£1£¬ŌņH2SO4ŗĶBa(OH)2·“Ó¦µÄ·“Ó¦ČȦ¤H£½2”Į(£57.3) kJ·mol£1(””””)
(4)Č¼ĮĻµē³ŲÖŠ½«¼×“¼ÕōĘų×Ŗ»ÆĪŖĒāĘųµÄČČ»Æѧ·½³ĢŹ½ŹĒCH3OH(g)£«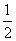 O2(g)===CO2(g)£«2H2(g)
O2(g)===CO2(g)£«2H2(g)
¦¤H£½£192.9 kJ·mol£1£¬ŌņCH3OH(g)µÄČ¼ÉÕČČĪŖ192.9 kJ·mol£1(””””)
(5)H2(g)µÄČ¼ÉÕČČŹĒ285.8 kJ·mol£1£¬Ōņ2H2O(g)===2H2(g)£«O2(g)””¦¤H£½571.6 kJ·mol£1(””””)
(6)ĘĻĢŃĢĒµÄČ¼ÉÕČČŹĒ2 800 kJ·mol£1£¬Ōņ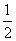 C6H12O6(s)£«3O2(g)===3CO2(g)£«3H2O(l)””¦¤H£½£1 400 kJ·mol£1(””””)
C6H12O6(s)£«3O2(g)===3CO2(g)£«3H2O(l)””¦¤H£½£1 400 kJ·mol£1(””””)
(7)ŅŃÖŖ101 kPaŹ±£¬2C(s)£«O2(g)===2CO(g)
¦¤H£½£221 kJ·mol£1£¬ŌņøĆ·“Ó¦µÄ·“Ó¦ČČĪŖ221 kJ·mol£1(””””)
(8)ŅŃÖŖĻ”ČÜŅŗÖŠ£¬H£«(aq)£«OH£(aq)===H2O(l)
¦¤H£½£57.3 kJ·mol£1£¬ŌņĻ”“×ĖįÓėĻ”ĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³É1 molĖ®Ź±·Å³ö57.3 kJµÄČČĮæ(””””)
(9)ŅŃÖŖHClŗĶNaOH·“Ó¦µÄÖŠŗĶČȦ¤H£½£57.3 kJ·mol£1£¬Ōņ98%µÄÅØĮņĖįÓėĻ”ĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³É1 molĖ®µÄÖŠŗĶČČĪŖ£57.3 kJ·mol£1(””””)
(10)CO(g)µÄČ¼ÉÕČČŹĒ283.0 kJ·mol£1£¬Ōņ2CO2(g)===2CO(g)£«O2(g)·“Ó¦µÄ¦¤H£½2”Į283.0 kJ·mol£1(””””)
(11)ĒāĘųµÄČ¼ÉÕČČĪŖ285.5 kJ·mol£1£¬Ōņµē½āĖ®µÄČČ»Æѧ·½³ĢŹ½ĪŖ2H2O(l)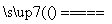 2H2(g)£«O2(g)””¦¤H£½285.5 kJ·mol£1(””””)
2H2(g)£«O2(g)””¦¤H£½285.5 kJ·mol£1(””””)

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ(””””)
A£®ÖÜĘŚ±ķÖŠµÄÖ÷×嶼ӊ·Ē½šŹōŌŖĖŲ
B£®ÖÜĘŚ±ķÖŠµÄÖ÷×嶼ӊ½šŹōŌŖĖŲ
C£®ÖÜĘŚ±ķÖŠµÄ·Ē½šŹōŌŖĖŲ¶¼Ī»ÓŚ¶ĢÖÜĘŚ
D£®ÖÜĘŚ±ķÖŠµÄ¹ż¶ÉŌŖĖŲ¶¼ŹĒ½šŹōŌŖĖŲ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹ŲÓŚ1s”¢2s”¢3s”¢4sŌ×Ó¹ģµĄµÄĖµ·Ø£¬ÕżČ·µÄŹĒ (””””)
A£®µē×ÓÖ»ÄÜŌŚµē×ÓŌĘĀÖĄŖĶ¼ÖŠŌĖ¶Æ
B£®¹ģµĄ²»Ķ¬£¬µē×ÓŌĘĀÖĄŖĶ¼ŠĪדĻąĶ¬
C£®¹ģµĄŹżÄæĻąĶ¬£¬µē×ÓŌĘĀÖĄŖĶ¼ŠĪד”¢“óŠ”ĶźČ«ĻąĶ¬
D£®ÄÜ²ć²»Ķ¬£¬µē×ÓŌĘĀÖĄŖĶ¼ŠĪדŅ²²»ĻąĶ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃé²āµĆ£ŗ101 kPaŹ±£¬1 mol H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®£¬·Å³ö285.8 kJµÄČČĮ棻1 mol CH4ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®ŗĶCO2£¬·Å³ö890.3 kJµÄČČĮ攣ĻĀĮŠČČ»Æѧ·½³ĢŹ½µÄŹéŠ“ÕżČ·µÄŹĒ(””””)
¢ŁCH4(g)£«2O2(g)===CO2(g)£«2H2O(l)
¦¤H£½890.3 kJ·mol£1
¢ŚCH4(g)£«2O2(g)===CO2(g)£«2H2O(l)
¦¤H£½£890.3 kJ·mol£1
¢ŪCH4(g)£«2O2(g)===CO2(g)£«2H2O(g)
¦¤H£½£890.3 kJ·mol£1
¢Ü2H2(g)£«O2(g)===2H2O(l)
¦¤H£½£571.6 kJ·mol£1
A£®½öÓŠ¢Ś B£®½öÓŠ¢Ś¢Ü C£®½öÓŠ¢Ś¢Ū¢Ü D£®Č«²æ·ūŗĻŅŖĒó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
LiHæÉ×÷·É“¬µÄČ¼ĮĻ£¬ŅŃÖŖĻĀĮŠ·“Ó¦£ŗ
¢Ł2Li(s)£«H2(g)===2LiH(s)””¦¤H£½£182 kJ·mol£1
¢Ś2H2(g)£«O2(g)===2H2O(l)””¦¤H£½£572 kJ·mol£1
¢Ū4Li(s)£«O2(g)===2Li2O(s)””¦¤H£½£1 196 kJ·mol£1
ŹŌŠ“³öLiHŌŚO2ÖŠČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖ»ŗ½āÄÜŌ“½ōÕÅ£¬Ō½Ą“Ō½¶ąµÄ¹ś¼ŅæŖŹ¼ÖŲŹÓÉśĪļÖŹÄÜŌ“(ĄūÓĆÄÜŌ“×÷ĪļŗĶÓŠ»ś·ĻĮĻ£¬¾¹ż¼Ó¹¤×Ŗ±äĪŖÉśĪļČ¼ĮĻµÄŅ»ÖÖÄÜŌ“)µÄæŖ·¢ĄūÓĆ”£
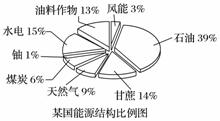
(1)ČēĶ¼ŹĒij¹śÄÜŌ“½į¹¹±ČĄżĶ¼£¬ĘäÖŠÉśĪļÖŹÄÜŌ“ĖłÕ¼µÄ±ČĄżŹĒ______”£
(2)ÉśĪļ²ńÓĶŹĒÓɶÆÖ²ĪļÓĶÖ¬×Ŗ»Æ¶ųĄ“£¬ĘäÖ÷ŅŖ³É·ÖĪŖÖ¬·¾Ėįõ„£¬¼øŗõ²»ŗ¬Įņ£¬ÉśĪļ½µ½āŠŌŗĆ£¬Ņ»Š©¹ś¼ŅŅŃ½«ĘäĢķ¼ÓŌŚĘÕĶزńÓĶÖŠŹ¹ÓĆ”£¹ŲÓŚÉśĪļ²ńÓĶ¼°ĘäŹ¹ÓĆ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________”£
¢ŁÉśĪļ²ńÓĶŹĒæÉŌŁÉś×ŹŌ“””¢ŚæɼõÉŁ¶žŃõ»ÆĮņµÄÅÅ·Å
¢ŪÓėĘÕĶزńÓĶĻą±ČŅ×·Ö½ā””¢ÜÓėĘÕĶزńÓĶÖĘČ”·½·ØĻąĶ¬
A£®¢Ł¢Ś¢Ū B£®¢Ł¢Ś¢Ü C£®¢Ł¢Ū¢Ü D£®¢Ś¢Ū¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĄūÓĆŗ¬Ģ¼»ÆŗĻĪļŗĻ³ÉČ¼ĮĻŹĒ½ā¾öÄÜŌ“Ī£»śµÄÖŲŅŖ·½·Ø£¬ŅŃÖŖCO(g)£«2H2(g)CH3OH(g)·“Ó¦¹ż³ĢÖŠµÄÄÜĮæ±ä»ÆĒéæöČēĶ¼ĖłŹ¾£¬ĒśĻߢńŗĶĒśĻߢņ·Ö±š±ķŹ¾²»Ź¹ÓĆ“ß»Æ¼ĮŗĶŹ¹ÓĆ“ß»Æ¼ĮµÄĮ½ÖÖĒéæö”£ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ(””””)
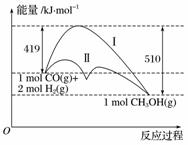
A£®øĆ·“Ó¦µÄ¦¤H£½91 kJ·mol£1
B£®¼ÓČė“߻ƼĮ£¬øĆ·“Ó¦µÄ¦¤H±äŠ”
C£®·“Ó¦ĪļµÄ×ÜÄÜĮæ“óÓŚÉś³ÉĪļµÄ×ÜÄÜĮæ
D£®Čē¹ūøĆ·“Ӧɜ³ÉŅŗĢ¬CH3OH£¬Ōņ¦¤HŌö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹéŠ“ÕżČ·µÄŹĒ(””””)
A£®½«AlĢõĶ¶ČėNaOHČÜŅŗÖŠ£ŗAl£«OH££«H2O===AlO £«H2”ü
£«H2”ü
B£®ĶČÜÓŚĻ”ĻõĖįÖŠ£ŗCu£«4H£«£«2NO ===Cu2£«£«2NO2”ü£«2H2O
===Cu2£«£«2NO2”ü£«2H2O
C£®Ģ¼ĖįĒāøĘČÜŅŗÖŠ¼ÓČė¹żĮæµÄĒāŃõ»ÆÄĘČÜŅŗ£ŗHCO £«OH£===CO
£«OH£===CO £«H2O
£«H2O
D£®ĻņĢ¼ĖįÄĘČÜŅŗÖŠÖšµĪ¼ÓČėÓėÖ®µČĢå»żµČĪļÖŹµÄĮæÅØ¶ČµÄĻ”“×Ėį£ŗCO £«CH3COOH===CH3COO££«HCO
£«CH3COOH===CH3COO££«HCO
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com