
£Ø14·Ö£©
I£®½öÓĆĻĀĮŠ×°ÖĆ£¬¼ģŃéľĢæŗĶÅØĮņĖį·“Ó¦²śÉśµÄ»ģŗĶĘųĢåÖŠŗ¬ÓŠSO2ŗĶCO2”£ŌŚĻĀĮŠ±ķøńÖŠĢīŠ“¶ŌÓ¦µÄŅ©Ę·ŗĶŹµŃéĻÖĻó”£
|
ŹµŃé×°ÖĆ |
¼ģŃéĘųĢå |
ŹŌ¼ĮĆū³Ę |
ŹµŃéĻÖĻó |
|
A |
SO2 |
|
|
|
B |
CO2 |
|
|
II£®ĄūÓĆĻĀĮŠ×°ÖĆĄ“µē½ā±„ŗĶŹ³ŃĪĖ®£¬²¢²āĮæµē½ā²śÉśµÄĒāĘųµÄĢå»ż£ØŌ¼6mL£©ŗĶ¼ģŃéĀČĘųµÄŃõ»ÆŠŌ£Ø²»Ó¦½«¶ąÓąµÄĀČĘųÅÅČėæÕĘųÖŠ£©”£
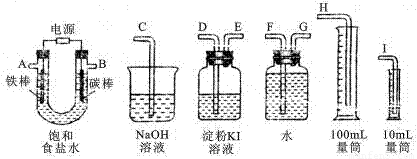
£Ø1£©½«ÉĻĆęø÷ŅĒĘ÷Į¬³ÉŅ»ÕūĢ××°ÖĆ£¬ø÷ÖÖŅĒĘ÷½ÓæŚµÄĮ¬½ÓĖ³Šņ£ØĢī±ąŗÅ£©ŹĒ£ŗA½Ó______________£¬B½Ó______________”£
£Ø2£©ĒėŠ“³öĢś°ōµē¼«ÉĻ·¢ÉśµÄµē¼«·“Ó¦Ź½__________________________”£
£Ø3£©ÄÜĖµĆ÷ĀČĘų¾ßÓŠŃõ»ÆŠŌµÄŹµŃéĻÖĻóŹĒ_______________________________”£
£Ø4£©ČōU¹Ü֊װČėµÄ±„ŗĶŹ³ŃĪĖ®ĪŖ50mL£Øµē½āĒ°ŗóČÜŅŗĢå»ż±ä»ÆæÉŗöĀŌ£©£¬µ±ŹÕ¼Æµ½µÄĒāĘųĪŖ5.6mL£Ø±ź×¼×“æö£©Ź±Ķ£Ö¹Ķصē£¬Ņ”ŌČUŠĶ¹ÜÄŚµÄČÜŅŗ£¬Č”20.00mLøĆČÜŅŗӌ׶ŠĪĘæÄŚ£¬ÓĆ0.01mol/LµÄŃĪĖįµĪ¶ØŹ±£¬µĪ¶Ø¹ÜĘšŹ¼¶ĮŹżĪŖ1.00mL£¬µĪ¶ØÖÕµćŹ±£¬µĪ¶Ø¹ÜµÄ¶ĮŹżĪŖ_______ mL”£
 ĆūŠ£ĆūŹ¦ÅąÓÅ×÷Ņµ±¾¼ÓŗĖŠÄŹŌ¾ķĻµĮŠ“š°ø
ĆūŠ£ĆūŹ¦ÅąÓÅ×÷Ņµ±¾¼ÓŗĖŠÄŹŌ¾ķĻµĮŠ“š°ø Č«³Ģ½š¾ķĻµĮŠ“š°ø
Č«³Ģ½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø13·Ö£©Ä³»ÆѧŠĖȤŠ”×éĪŖĢ½Ė÷ĶøśÅØĮņĖįµÄ·“Ó¦£¬ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠÓŠ¹ŲŹµŃ锣Ēė»Ų“š£ŗ
I£®ŹµŃéÖŠ¼×Ķ¬Ń§Č”a æĖ Cu ʬŗĶ12ml18mol/LÅØH2SO4·ÅČĖŌ²µ×ÉÕĘæÖŠ¼ÓČČ£¬Ö±µ½·“Ó¦Ķź±Ļ£¬×īŗó·¢ĻÖÉÕĘæÖŠ»¹ÓŠŅ»¶ØĮæµÄH2SO4ŗĶCuŹ£Óą”£
£Ø1£©ĒėŠ“³öCuÓėÅØH2SO4·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
×°ÖĆEÖŠŹŌ¹ÜDÄŚŹ¢Ę·ŗģČÜŅŗ£¬µ±CÖŠĘųĢå¼ÆĀśŗó£¬DÖŠÓŠæÉÄܹŪ²ģµ½µÄĻÖĻóŹĒ__________”£
ŹµŃé×°ÖĆDÓŠæÉÄÜŌģ³É»·¾³ĪŪČ¾£¬ŹŌÓĆ×ī¼ņµ„·½·Ø¼ÓŅŌ½ā¾ö£ØŹµŃéÓĆĘ·×ŌŃ”£©_________”£
£Ø2£©×°ÖĆBµÄ×÷ÓĆŹĒÖü“궹ӹµÄĘųĢ唣µ±D“¦ÓŠĆ÷ĻŌĻÖĻóŗ󣬹Ų±ÕŠżČūK£¬ŅĘČ„¾Ę¾«µĘ£¬µ«ÓÉÓŚÓąČȵÄ×÷ÓĆ£¬A“¦ČŌÓŠĘųĢå²śÉś½ųČėB£¬BÖŠÓ¦·ÅÖƵÄŅŗĢå£ØĢī×ÖÄø£©£ŗ________
A£®±„ŗĶNa2SO3ČÜŅŗ B£®ĖįŠŌ KMnO4ČÜŅŗ
C£®ÅØäåĖ® D£®±„ŗĶNaHSO3ČÜŅŗ
£Ø3£©ĪŹĢāĢÖĀŪ£ŗĪŖŹ²Ć“ÓŠŅ»¶ØĮæµÄÓąĖįµ«Ī“ÄÜŹ¹CuĶźČ«ČܽāÄŲ?
×ćĮæĻĀĮŠŅ©Ę·Äܹ»ÓĆĄ“Ö¤Ć÷·“Ó¦½įŹųŗóµÄÉÕĘæÖŠµÄČ·ÓŠÓąĖįµÄŹĒ_________”£
A£®Fe·Ū B£®BaCl2ČÜŅŗ C£®Ag D£®±„ŗĶNaHSO3ČÜŅŗ
£Ø4£©ŹµŃéÖŠ¼×ѧɜĻņAÖŠ·“Ó¦ŗóČÜŅŗÖŠĶØČėŅ»ÖÖ³£¼ūĘųĢåµ„ÖŹ£¬Ź¹Ķʬȫ²æČܽāĒŅ½öÉś³ÉĮņĖįĶČÜŅŗ£¬ĒėĪŹøĆĘųĢåµ„ÖŹŹĒ___ £ØĢīĆū³Ę£©£¬·“Ó¦·½³ĢŹ½ŹĒ______ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø14·Ö£©
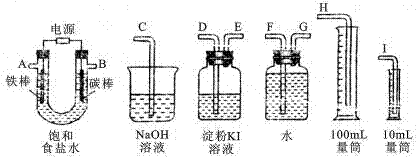
I£®½öÓĆĻĀĮŠ×°ÖĆ£¬¼ģŃéľĢæŗĶÅØĮņĖį·“Ó¦²śÉśµÄ»ģŗĶĘųĢåÖŠŗ¬ÓŠSO2ŗĶCO2”£ŌŚĻĀĮŠ±ķøńÖŠĢīŠ“¶ŌÓ¦µÄŅ©Ę·ŗĶŹµŃéĻÖĻó”£
| ŹµŃé×°ÖĆ | ¼ģŃéĘųĢå | ŹŌ¼ĮĆū³Ę | ŹµŃéĻÖĻó |
| A | SO2 |
|
|
| B | CO2 |
|
|
II£®ĄūÓĆĻĀĮŠ×°ÖĆĄ“µē½ā±„ŗĶŹ³ŃĪĖ®£¬²¢²āĮæµē½ā²śÉśµÄĒāĘųµÄĢå»ż£ØŌ¼6mL£©ŗĶ¼ģŃéĀČĘųµÄŃõ»ÆŠŌ£Ø²»Ó¦½«¶ąÓąµÄĀČĘųÅÅČėæÕĘųÖŠ£©”£
£Ø1£©½«ÉĻĆęø÷ŅĒĘ÷Į¬³ÉŅ»ÕūĢ××°ÖĆ£¬ø÷ÖÖŅĒĘ÷½ÓæŚµÄĮ¬½ÓĖ³Šņ£ØĢī±ąŗÅ£©ŹĒ£ŗA½Ó______________£¬B½Ó______________”£
£Ø2£©ĒėŠ“³öĢś°ōµē¼«ÉĻ·¢ÉśµÄµē¼«·“Ó¦Ź½__________________________”£
£Ø3£©ÄÜĖµĆ÷ĀČĘų¾ßÓŠŃõ»ÆŠŌµÄŹµŃéĻÖĻóŹĒ_______________________________”£
£Ø4£©ČōU¹Ü֊װČėµÄ±„ŗĶŹ³ŃĪĖ®ĪŖ50mL£Øµē½āĒ°ŗóČÜŅŗĢå»ż±ä»ÆæÉŗöĀŌ£©£¬µ±ŹÕ¼Æµ½µÄĒāĘųĪŖ5.6mL£Ø±ź×¼×“æö£©Ź±Ķ£Ö¹Ķصē£¬Ņ”ŌČUŠĶ¹ÜÄŚµÄČÜŅŗ£¬Č”20.00mLøĆČÜŅŗӌ׶ŠĪĘæÄŚ£¬ÓĆ0.01mol/LµÄŃĪĖįµĪ¶ØŹ±£¬µĪ¶Ø¹ÜĘšŹ¼¶ĮŹżĪŖ1.00mL£¬µĪ¶ØÖÕµćŹ±£¬µĪ¶Ø¹ÜµÄ¶ĮŹżĪŖ_______ mL”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğø£½ØŹ”ĖĵŲĮłŠ£øßŅ»ĻĀѧʌµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£Ø13·Ö£©Ä³»ÆѧŠĖȤŠ”×éĪŖĢ½Ė÷ĶøśÅØĮņĖįµÄ·“Ó¦£¬ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠÓŠ¹ŲŹµŃ锣Ēė»Ų“š£ŗ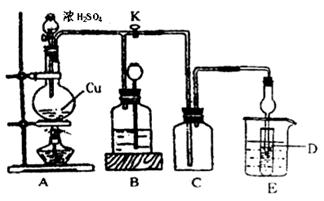
I£®ŹµŃéÖŠ¼×Ķ¬Ń§Č”a æĖ Cu ʬŗĶ12ml 18mol/LÅØH2SO4·ÅČĖŌ²µ×ÉÕĘæÖŠ¼ÓČČ£¬Ö±µ½·“Ó¦Ķź±Ļ£¬×īŗó·¢ĻÖÉÕĘæÖŠ»¹ÓŠŅ»¶ØĮæµÄH2SO4ŗĶCuŹ£Óą”£
£Ø1£©ĒėŠ“³öCuÓėÅØH2SO4·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
×°ÖĆEÖŠŹŌ¹ÜDÄŚŹ¢Ę·ŗģČÜŅŗ£¬µ±CÖŠĘųĢå¼ÆĀśŗó£¬DÖŠÓŠæÉÄܹŪ²ģµ½µÄĻÖĻóŹĒ__________”£
ŹµŃé×°ÖĆDÓŠæÉÄÜŌģ³É»·¾³ĪŪČ¾£¬ŹŌÓĆ×ī¼ņµ„·½·Ø¼ÓŅŌ½ā¾ö£ØŹµŃéÓĆĘ·×ŌŃ”£©_________”£
£Ø2£©×°ÖĆBµÄ×÷ÓĆŹĒÖü“궹ӹµÄĘųĢ唣µ±D“¦ÓŠĆ÷ĻŌĻÖĻóŗ󣬹Ų±ÕŠżČūK£¬ŅĘČ„¾Ę¾«µĘ£¬µ«ÓÉÓŚÓąČȵÄ×÷ÓĆ£¬A“¦ČŌÓŠĘųĢå²śÉś½ųČėB£¬BÖŠÓ¦·ÅÖƵÄŅŗĢå£ØĢī×ÖÄø£©£ŗ________
A£®±„ŗĶNa2SO3ČÜŅŗ B£®ĖįŠŌ KMnO4ČÜŅŗ
C£®ÅØäåĖ® D£®±„ŗĶNaHSO3ČÜŅŗ
£Ø3£©ĪŹĢāĢÖĀŪ£ŗĪŖŹ²Ć“ÓŠŅ»¶ØĮæµÄÓąĖįµ«Ī“ÄÜŹ¹CuĶźČ«ČܽāÄŲ?
×ćĮæĻĀĮŠŅ©Ę·Äܹ»ÓĆĄ“Ö¤Ć÷·“Ó¦½įŹųŗóµÄÉÕĘæÖŠµÄČ·ÓŠÓąĖįµÄŹĒ_________”£
A£®Fe·Ū B£®BaCl2ČÜŅŗ C£®Ag D£®±„ŗĶNaHSO3ČÜŅŗ
£Ø4£©ŹµŃéÖŠ¼×ѧɜĻņAÖŠ·“Ó¦ŗóČÜŅŗÖŠĶØČėŅ»ÖÖ³£¼ūĘųĢåµ„ÖŹ£¬Ź¹Ķʬȫ²æČܽāĒŅ½öÉś³ÉĮņĖįĶČÜŅŗ£¬ĒėĪŹøĆĘųĢåµ„ÖŹŹĒ___ £ØĢīĆū³Ę£©£¬·“Ó¦·½³ĢŹ½ŹĒ______ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģŗž±±Ź”°ĖŹŠøßČż3ŌĀĮŖæ¼ĄķæĘ×ŪŗĻŹŌ¾ķ£Ø»Æѧ²æ·Ö£© ĢāŠĶ£ŗŹµŃéĢā
£Ø14·Ö£©
I£®½öÓĆĻĀĮŠ×°ÖĆ£¬¼ģŃéľĢæŗĶÅØĮņĖį·“Ó¦²śÉśµÄ»ģŗĶĘųĢåÖŠŗ¬ÓŠSO2ŗĶCO2”£ŌŚĻĀĮŠ±ķøńÖŠĢīŠ“¶ŌÓ¦µÄŅ©Ę·ŗĶŹµŃéĻÖĻó”£
| ŹµŃé×°ÖĆ | ¼ģŃéĘųĢå | ŹŌ¼ĮĆū³Ę | ŹµŃéĻÖĻó |
| A | SO2 | | |
| B | CO2 | | |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com