
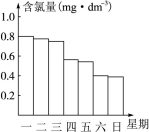
| A����ʪ���ë����ס�ڱ�����ʹ� |
| B���ý�ʪС�մ�����ˮ��ë����ס�ڱ�����ߴ� |
| C���ý�ʪŨ��ˮ��ë����ס�ڱ�����������ȫ�� |
| D���ý�ʪʳ��ˮ��ë����ס�ڱ�˳��������ȫ�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ҵ��������ͨ�˴�����Һ������Ư�� |
B����ҵ����������������У���Ԫ�ص�ת�������ǣ�FeS2 SO3 SO3 H2SO4 H2SO4 |
| C����¯��������Ҫ��Ӧԭ����̼�ڸ�����ֱ�ӽ���������ԭ���� |
| D���ں�ˮ�м���ʯ����ɵ�������þ���������ǴӺ�ˮ�и���þ�Ļ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
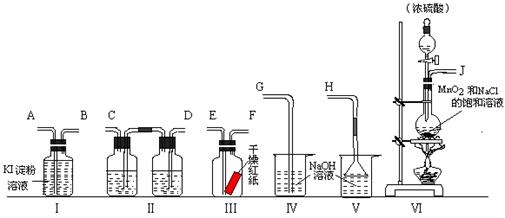
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����=��>�� | B����>��>�� | C����>��=�� | D����>��>�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ClO3-��Cl����K�� | B��ClO����Cl����H�� |
| C��NaClO��NaClO3��NaNO3 | D��NaClO��Na2SO4��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ף��ң��� | B���ף������� | C�������ף��� | D���ң������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
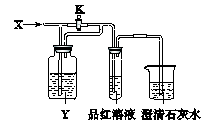
| | A | B | C | D |
| X | SO2 | NO2 | HCl | Cl2 |
| Y | ����NaHCO3��Һ | ˮ | Na2SO3��Һ | Na2SO3��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com