
| ���� | CH3COOH | HCN | H2CO3 |
| Ka | 1.8��10-5 | 4.9��10-10 | K1��4.3��10-7 K2��5.6��10-11 |
| A��pH��ͬ��������Һ���ʵ���Ũ�ȹ�ϵ��c��Na2CO3����c��NaCN����c��CH3COONa�� |
| B��a L 0.1mol?L-1CH3COOH��b L 0.1mol?L-1NaOH��Һ��Ϻ�������Һ��pH��7����aһ��С�ڻ����b |
| C�����ʵ���Ũ�Ⱥ��������ͬ��CH3COONa��NaOH������Һ����֪ǰ����Һ��������ĿΪn1��������Һ��������ĿΪn2����n1��n2 |
| D�����ʵ���Ũ����ͬNaHCO3��Na2CO3�Ļ����Һ�У�2c��H+��-2c��OH-��=c��CO32-��-c��HCO3-��-c��H2CO3�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
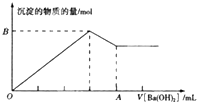 ��֪Ba[Al��OH��4]2������ˮ��ͼ�б�ʾ����100mL0.02mol��L-1KAl��SO4��2��Һ�������0.05mol��L-1Ba��OH��2��Һʱ��25�棩�����ɳ��������ʵ��������Ba��OH��2��Һ������Ĺ�ϵ������˵������ȷ���ǣ�������
��֪Ba[Al��OH��4]2������ˮ��ͼ�б�ʾ����100mL0.02mol��L-1KAl��SO4��2��Һ�������0.05mol��L-1Ba��OH��2��Һʱ��25�棩�����ɳ��������ʵ��������Ba��OH��2��Һ������Ĺ�ϵ������˵������ȷ���ǣ�������| A�����۵�Ba��OH��2��Һ��c��OH-��=0.1mol��L- |
| B��A���ֵ��80mL |
| C��B���ֵ��0.005mol |
| D����V[Ba��OH��2]ʱ�����ɳ�����������0.699g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����������Ե���� |
| B��Һ������������� |
| C���Ȼ��Ƴ������к�θ�� |
| D����������������ʢװϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ۢ� | C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��150mL 1mol/L NaCl |
| B��75mL 2mol/L NH4Cl |
| C��150mL 3mol/L KClO3 |
| D��75mL 1.5mol/L MgCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Zn��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 5.4 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 8.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
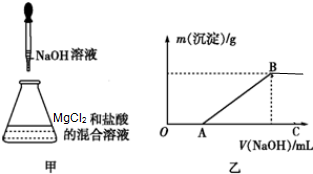 ijͬѧ��ȡһ������MgCl2���������Һ���ڸ���Һ���ȼ���һ������ϡ���ᣬȻ����μ���NaOH��Һ����ͼ����ʾ��
ijͬѧ��ȡһ������MgCl2���������Һ���ڸ���Һ���ȼ���һ������ϡ���ᣬȻ����μ���NaOH��Һ����ͼ����ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �Ե������Ǵӷ仨��ֲ������ȡ�õ����������ʣ���ṹ����ͼ������������ȷ���ǣ�������
�Ե������Ǵӷ仨��ֲ������ȡ�õ����������ʣ���ṹ����ͼ������������ȷ���ǣ�������| A���Ե��������ڷ����� |
| B��1mol�Ե���������ܺ�1mol���������ӳɷ�Ӧ |
| C���Ե�������Է���ˮ�ⷴӦ�����ܷ���������Ӧ |
| D���Ե�������ֻ��һ������̼ԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com