
| ����ǰ���� | ���Ⱥ����� | ||
| m1 | m2 | m3 | m4 |
| 5.400g | 7.900g | 6.900g | 6.901g |
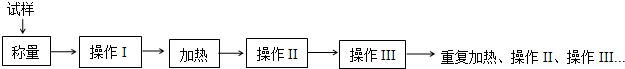
���� ��1���ⶨFeSO4•xH2O�нᾧˮ������ʵ������������ȡ���ȴ�����������أ������������н��У�����Ҫ�����ǡ����Ǽܡ��ƾ��ơ��в�������ǯ����������ҩ�ף��ڸ���������ȴ�����õ�����ƽ���������������
��2�����ݽᾧˮ�����У��ᾧˮ������=m������ʮ���壩-m������ʮ��ˮ����ͭ�����ݻ�ѧ����ʽ���Լ���ᾧˮx��ֵ��
��3���ڲⶨ����������Ʒ�к��м��Ȼӷ������ʻ�ʵ��ǰ��������ˮ��������ɲ������ƫ�ߡ�ƫ�ͣ�
��� �⣺��1��ʵ������������������ƽ���ƾ��ơ������żܣ�������������ѡ��ʵ�黹��Ҫ������A��B��E��F��G�ȣ��������г��������⣬����Ҫ�������������ǡ�����ǯ���ʴ�Ϊ��A��B��E��F��G�������ǡ�����ǯ��
��2���ɱ����е����ݣ�����ͭ���������Ϊm2-m1=7.900g-5.400g=2.500g��ˮ������Ϊ7.900g-6.900g=1.000g��
��CuSO 4��xH2O$\frac{\underline{\;\;��\;\;}}{\;}$CuSO 4+xH2O��
160+18x 18x
2.500g 1.000g
$\frac{160+18x}{2.500}$=$\frac{18x}{1.000}$�����x=5.9��
�ʴ�Ϊ��5.9��
��3��ijѧ����ʵ������x=5.64�����ѧ������ʵ�����ƫС��
a������ǰ����ʱ����δ��ȫ���ˮ������ƫ��xƫ�ʲ�ѡ��
b��������μ��Ⱥ���������ϴ���û�зֽ���ȫ��ˮ������ƫС��xƫС����ѡ��
c�����Ⱥ�����δ�������������ȴ��������ƫС��ˮ������ƫС��xƫС����ѡ��
d�����ȹ�������������ʧ��ʵ��ʧ�ܣ���ʹ��õĽᾧˮ������ƫ��ʹʵ����ƫ�ߣ��ʲ�ѡ��
�ʴ�Ϊ��bc��
���� ���⿼�����ʺ����IJⶨʵ�飬Ϊ��Ƶ���㣬���ղⶨԭ����ʵ��������ʹ�á�ʵ�鼼�ܵ�Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��ᾧˮ����������������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
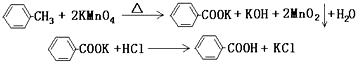
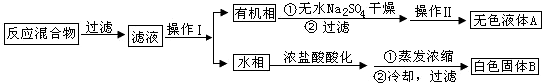
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���¡���ѹ������ | B�� | ���¡���ѹ������ | ||
| C�� | ���¡���ѹ������ | D�� | �����¶ȡ���ѹ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| Ԫ�� | I1 | I2 | I3 | I4 |
| X | 500 | 4600 | 6900 | 9500 |
| Y | 580 | 1820 | 2750 | 11600 |
| A�� | Ԫ��X�ij������ϼ���+3 | |
| B�� | Ԫ��Y�Ǣ�A��Ԫ�� | |
| C�� | Ԫ��X����Ԫ���γɻ�����ʱ����ѧʽ������XCl2 | |
| D�� | ��Ԫ��Y���ڵ������ڣ���������ˮ���ҷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

| �ŵ�Ʒ | һ��Ʒ | �ϸ�Ʒ | |
| ��� | ��ɫ�ᾧ���ɼ���е���� | �ɼ���е���� | |
| ����N������ | ��21.0% | ��21.0% | ��20.5% |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ����������g�� | �����;������������g�� | ���Ⱥ�������ʣ������������g�� |
| 11.685 | 13.691 | 12.948 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2��NO2��CO2�Ĵ����ŷŶ��ᵼ��������γ� | |
| B�� | �������Ȼ�����Ĵ����ŷŻ��ƻ����������� | |
| C�� | �ؽ������л�ũҩ��ҵ��ˮ�������ŷŵȻ����ˮ����Ⱦ | |
| D�� | װ�β����еļ�ȩ�����ͷ�����Ԫ��뱵Ȼ���ɾ�����Ⱦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
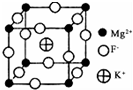 ±��Ԫ���ǵ��͵ķǽ���Ԫ�أ����ʼ��仯�����ڹ�ũҵ����������������Ҫ����;��
±��Ԫ���ǵ��͵ķǽ���Ԫ�أ����ʼ��仯�����ڹ�ũҵ����������������Ҫ����;�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
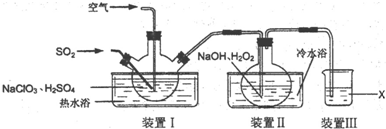
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com