
ijͬѧ��̽��ʳƷ���Ӽ������NH4Al(SO4)2��12H2O���·ֽ�������
��1��Ԥ�������й�����������Ԥ������������?????? ��
A��NH3��N2��SO2��H2O?????????? B��NH3��SO3��H2O
C��NH3��SO2��H2O?????????????? D��NH3��N2��SO3��SO2��H2O
��2�����Լ��飺ȡһ������������������ʵ��̽�����
����ͼʾ��װ���������ȼ������װ�õ������ԣ�������???????? ?????????? ��
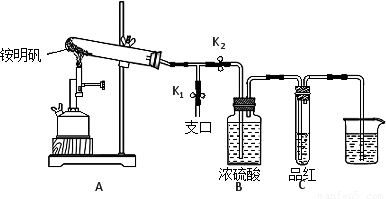
����סֹˮ��K1����ֹˮ��K2���þƾ���Ƴ�����ա�ʵ������У�װ��A�͵�����δ������ɫ���壻�Թ�C�е�Ʒ����Һ��ɫ����֧�ڴ��ɼ��鵽NH3��������????? ??????????? ����װ��A��B֮���T�͵����г��ְ�ɫ���壬�ð�ɫ���������???? ?? ������һ�����ʵĻ�ѧʽ����
�������ó�װ��A�Թ��в����İ�ɫ���������������д��������NaOH��Һ�����ӷ���ʽ?????? ??????????????????????????????? ��
��Ϊ�˷�ֹ������ʵ�����ʱ������???? ?? ������ĸ��ţ���Ȼ��Ϩ��ƾ���ơ�
A��ȡ���ձ��еĵ���????? B����ֹˮ��K1??????? C���ر�ֹˮ��K2
��3�������ͽ��ۣ�ʵ��֤����������ǣ�1��D�е�5�����塣��ͬ�����²������N2��SO2��������Ƕ�ֵ��V��N2����V��SO2��=?????????? ��
��1��C��2�֣�
��2�����ر�֧�ڿ���K1����K2��1�֣��������ĵ���ͨ��ˮ�У��ȴ��Թ���1�֣����������ӵ����г���������1�֣�����ֹͣ���Ⱥ��ڵ���������һ��ˮ������1�֣���֤�������Ժ�������4�֣�
�ڴ�K1����պ��Ũ����IJ���������֧�ڣ������ְ��̣����߲����Լ���������Ҳ���֣���2�֣�������ʪ����ɫʯ����ֽ���鲻�÷֣���(NH4)2SO4����(NH4)2SO3����SO3(��ʽ�μ��������ʵĻ����Ҳ����)��2�֣�
��Al2O3 +2OH-=2AlO2-+H2O��Al2O3 +3H2O +2OH-=2Al(OH)4-��2������ƽ�����0����
��B��C����BC��2�֣�
��3��1:3��2�֣�
��������
�����������1��A������N2��NԪ�ػ��ϼ����ߣ�����SO2��SԪ�ػ��ϼ۽��ͣ�����������ԭ��Ӧԭ����Ԥ�������B������NH3��SO3��H2O��Ϊ��������ԭ��Ӧ��Ԥ�������C������SO2��SԪ�ػ��ϼ۽��ͣ���Ԫ�ػ��ϼ����ߣ�������������ԭ��Ӧԭ����Ԥ�ⲻ������D������Ԫ�ػ��ϼ����ߣ�Ҳ��Ԫ�ػ��ϼ۽��ͣ�Ԥ�������
��2�������ü����������͵�ԭ������װ�õ������ԣ�����Ҫ�ر�֧�ڿ���K1����K2��Ȼ�������ĵ���ͨ��ˮ�У��ȴ��Թ����������ӵ����г������ݣ���ֹͣ���Ⱥ��ڵ���������һ��ˮ������֤�������Ժ���
����Ũ�������NH3����K1����պ��Ũ����IJ���������֧�ڣ������ְ�����֤������NH3������������ʪ��ʯ����ֽ����Ϊ��������к���SO2��SO3����ֽ�����ܱ�����װ��A��B֮���T�͵����г��ְ�ɫ����������SO2��NH3��Ӧ���ɵ�(NH4)2SO3����SO3��NH3��Ӧ���ɵ�(NH4)2SO4����SO3���壬����ʽ�μ��������ʵĻ������
��A�Թ��в����İ�ɫ�����������������AΪAl2O3����NaOH��Һ��Ӧ�����ӷ���ʽΪ��Al2O3 +2OH-=2AlO2-+H2O��Al2O3 +3H2O +2OH-=2Al(OH)4-��
����ֹˮ��K1 ������Һ����֧�ڳ��������ر�ֹˮ��K2�����ܱ��رգ����Է�ֹ�����ķ���Ϊ��B��C����BC��
��3������������ԭ��Ӧ�л��ϼ����ߵ��ܼ����뽵�͵��ܼ�����ȣ��ɵã�6n(N2)=2n(SO2)���ɵ� n(N2)��n(SO2)=1:3����ͬ�������������ʵ���֮�ȵ������֮�ȣ�����V��N2����V��SO2��=1:3��???
���㣺���⿼��ʵ�鷽��������������������ԭ��Ӧԭ�������㡢�������������ӷ���ʽ����д��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�� | ʵ������ | ��Ӧ���ӷ���ʽ�ͽ��� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ��Һ�п϶�����Fe2+��Mg2+��SiO32- | B�����������ɫ������ܺ���CO2��ԭ��Һ�п��ܺ���CO32- | C��ԭ��Һ�п϶�����K+��Fe2+��NO3-��SO42-��I- | D��Ϊȷ���Ƿ���Cl-����ȡԭ��Һ����������������ϡ���ᣬ�۲��Ƿ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com