
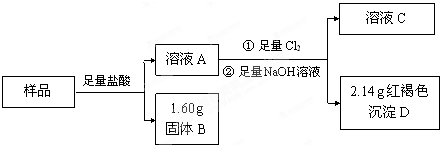
| 2.14g |
| 107g/mol |
| 2.28g |
| 5.00g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����³�ѹ�£�1mol��ϩ���õ��Ӷ���Ϊ4NA |
| B��1mol��ȩ����������Cu��OH��2����Һ��Ӧ��ת�Ƶ�����ĿΪNA |
| C����״���£�1L������ȼ�պ�������̬����ķ�����Ϊ5/22.4 NA |
| D��0.1mol��ϩ���Ҵ��Ļ������ȫȼ�������ĵ���ԭ����һ��Ϊ0.6 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
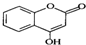 ��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�
��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�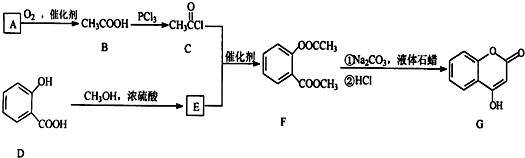
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
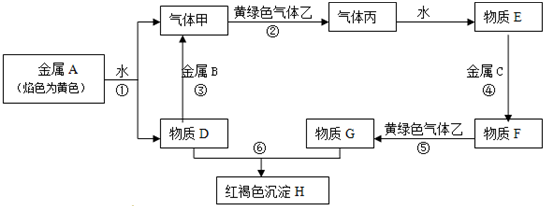
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ŨH2SO4 |
| �� |
| Br2 |
| CCl4 |
| A��CH3CH2CHBrCH2Br |
| B��CH3CH��CH2Br��2 |
| C��CH3CHBrCHBrCH3 |
| D����CH3��2CBrCH2Br |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
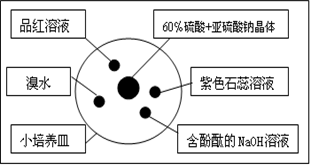
| A����ˮ��ɫ��ȥ��ԭ����SO2�������Ư���� |
| B������̪��NaOH��Һ��ɫ��dz��ԭ����SO2�������Ư���� |
| C��Ʒ����Һ��ɫ��ԭ����SO2�������Ư���� |
| D����ɫʯ����Һ����ɫ��ԭ����SO2����������Ժ�Ư���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| A��7CH4��g��+3O2��g��+H2O��g��=7CO��g��+15H2��g�� |
| B��5CH4��g��+2O2��g��+H2O��g��=5CO��g��+11H2��g�� |
| C��4CH4��g��+O2��g��+2H2O��g��=4CO��g��+10H2��g�� |
| D��3CH4��g��+O2��g��+H2O��g��=3CO��g��+7H2��g�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com