
¶ĢÖÜĘŚŌŖĖŲµÄµ„ÖŹX”¢Y”¢ZŌŚĶس£×“æöĻĀ¾łĪŖĘųĢ¬£¬²¢ÓŠĻĀĮŠ×Ŗ»Æ¹ŲĻµ£Ø·“Ó¦Ģõ¼žĀŌČ„£©£ŗ
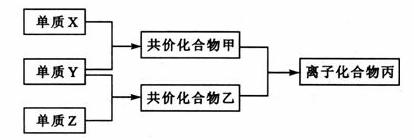
ŅŃÖŖ£ŗa.³£¼ūĖ«Ō×Óµ„ÖŹ·Ö×ÓÖŠ£¬X·Ö×Óŗ¬¹²¼Ū¼ü×ī¶ą”£
b.¼×·Ö×Óŗ¬10øöµē×Ó£¬ŅŅ·Ö×Óŗ¬18øöµē×Ó”£
£Ø1£©XµÄµē×ÓŹ½ŹĒ______________________________”£
£Ø2£©ŹµŃéŹŅæÉÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ£ØȱɣŹÕ¼Æ×°ÖĆ£¬¼Š³Ö¹Ģ¶Ø×°ÖĆĀŌČ„£©Öʱø²¢ŹÕ¼Æ¼×”£

¢ŁŌŚĶ¼ÖŠ·½æņÄŚ»ę³öÓĆÉÕĘæŹÕ¼Æ¼×µÄŅĒĘ÷×°ÖĆ¼ņĶ¼”£
¢ŚŹŌ¹ÜÖŠµÄŹŌ¼ĮŹĒ£ØĢīŠ“»ÆѧŹ½£©________________________________________”£
¢ŪÉÕ±ÖŠČÜŅŗÓÉĪŽÉ«±äĪŖŗģÉ«£¬ĘäŌŅņŹĒ£ØÓƵēĄė·½³ĢŹ½±ķŹ¾£©
______________________________________________________________________ӣ
£Ø3£©Į×ŌŚZÖŠČ¼ÉÕæÉÉś³ÉĮ½ÖÖ²śĪļ£¬ĘäÖŠŅ»ÖÖ²śĪļ¶”·Ö×ÓÖŠø÷Ō×Ó×īĶā²ć²»Č«ŹĒ8µē
×Ó½į¹¹£¬¶”µÄ»ÆѧŹ½ŹĒ____________________”£
£Ø4£©n mol¶”Óėn mol±ūŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦£¬Éś³É4n molŅŅŗĶĮķŅ»»ÆŗĻĪļ£¬øĆ»ÆŗĻĪļÕōĘųµÄĆܶȏĒĻąĶ¬×“æöĻĀĒāĘųµÄ174±¶£¬Ęä»ÆѧŹ½ŹĒ____________________”£
£Ø1£©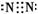
£Ø2£©¢Ł
¢ŚNH4Cl”¢Ca£ØOH£©2””£ØŗĻĄķ¾łæÉ£©
¢ŪNH3”¤H2O
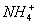 +OH-
+OH-
£Ø3£©PCl5
£Ø4£©P3N3Cl6
”¾½āĪö”æ±¾ĢāŹĒŅ»µĄĪŽ»ś»ÆѧĶʶĻĢā”£ÓÉĢāŅāÖŖ¼×ĪŖNH3£¬ŅŅĪŖHCl£¬XĪŖN2£¬YĪŖH2£¬ZĪŖCl2”£ŅņĪŖNH3¼«Ņ×ČÜÓŚĖ®£¬ĆܶȱČæÕĘųŠ”£¬ÓĆĻņĻĀÅÅĘų·ØŹÕ¼Æ”£2NH4Cl+Ca(OH)2 CaCl2+2NH3”ü+2H2O£¬°±Ė®³Ź¼īŠŌ£¬µĪČė·ÓĢŖČÜŅŗ±äŗģ£¬NH3”¤H2O
CaCl2+2NH3”ü+2H2O£¬°±Ė®³Ź¼īŠŌ£¬µĪČė·ÓĢŖČÜŅŗ±äŗģ£¬NH3”¤H2O
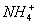 +OH£”£Į×ŌŚCl2ÖŠČ¼ÉÕÉś³ÉPCl3ŗĶPCl5£¬Óɶ”·Ö×Ó×īĶā²ć²»Č«ŹĒ8µē×Ó½į¹¹ÖŖ¶”ĪŖPCl5”£øĆ»ÆŗĻĪļÕōĘųµÄĆܶȏĒĻąĶ¬×“æöĻĀĒāĘųµÄ174±¶£¬¹ŹĻą¶Ō·Ö×ÓÖŹĮæĪŖ174”Į2=348£¬ÓÉĢā
+OH£”£Į×ŌŚCl2ÖŠČ¼ÉÕÉś³ÉPCl3ŗĶPCl5£¬Óɶ”·Ö×Ó×īĶā²ć²»Č«ŹĒ8µē×Ó½į¹¹ÖŖ¶”ĪŖPCl5”£øĆ»ÆŗĻĪļÕōĘųµÄĆܶȏĒĻąĶ¬×“æöĻĀĒāĘųµÄ174±¶£¬¹ŹĻą¶Ō·Ö×ÓÖŹĮæĪŖ174”Į2=348£¬ÓÉĢā ŅāøĆ»ÆŗĻĪļÖŠN”¢PøöŹżĻąĶ¬£¬ÉčĪŖ(NP)xCly
ŅāøĆ»ÆŗĻĪļÖŠN”¢PøöŹżĻąĶ¬£¬ÉčĪŖ(NP)xCly
45”¤x+35.5”¤y=348 x=3 y=6
»ÆѧŹ½ĪŖP3N3Cl6
·“Ó¦Ź½ĪŖ3PCl5+3NH4Cl P3N3Cl6+12HCl
P3N3Cl6+12HCl



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
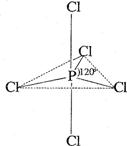 °×Į×£ØP4£©ŹĒĮ׵ĵ„ÖŹÖ®Ņ»£¬Ņ×Ńõ»Æ£¬ÓėĀ±ĖŲµ„ÖŹ·“Ӧɜ³ÉĀ±»ÆĮ×”£Ā±»ÆĮ×Ķس£ÓŠČżĀ±»ÆĮ×»ņĪåĀ±»ÆĮ×£¬ĪåĀ±»ÆĮ×·Ö×Ó½į¹¹£ØŅŌPCl5ĪŖĄż£©ČēÓŅĶ¼ĖłŹ¾”£øĆ½į¹¹ÖŠĀČŌ×ÓÓŠĮ½ÖÖ²»Ķ¬Ī»ÖĆ”£
°×Į×£ØP4£©ŹĒĮ׵ĵ„ÖŹÖ®Ņ»£¬Ņ×Ńõ»Æ£¬ÓėĀ±ĖŲµ„ÖŹ·“Ӧɜ³ÉĀ±»ÆĮ×”£Ā±»ÆĮ×Ķس£ÓŠČżĀ±»ÆĮ×»ņĪåĀ±»ÆĮ×£¬ĪåĀ±»ÆĮ×·Ö×Ó½į¹¹£ØŅŌPCl5ĪŖĄż£©ČēÓŅĶ¼ĖłŹ¾”£øĆ½į¹¹ÖŠĀČŌ×ÓÓŠĮ½ÖÖ²»Ķ¬Ī»ÖĆ”£
1£©6.20g°×Į×ŌŚ×ćĮæŃõĘųÖŠĶźČ«Č¼ÉÕÉś³ÉŃõ»ÆĪļ£¬·“Ó¦ĖłĻūŗĵÄŃõĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ L”£
ÉĻŹöČ¼ÉÕ²śĪļČÜÓŚĖ®Åä³É50.0mLĮ×Ėį£ØH3PO4£©ČÜŅŗ£¬øĆĮ×ĖįČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ mol”¤L-1”£
2£©ŗ¬0.300mol H3PO4µÄĖ®ČÜŅŗµĪ¼Óµ½ŗ¬0.500mol Ca(OH)2µÄŠüø”ŅŗÖŠ£¬·“Ó¦Ē”ŗĆĶźČ«£¬Éś³ÉlÖÖÄŃČÜŃĪŗĶ16.2g H2O”£øĆÄŃČÜŃĪµÄ»ÆѧŹ½æɱķŹ¾ĪŖ ”£
3£©°×Į×ŗĶĀČ”¢äå·“Ó¦£¬Éś³É»ģŗĻĀ±»ÆĮ×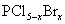 £Ø
£Ø £¬ĒŅxĪŖÕūŹż£©”£
£¬ĒŅxĪŖÕūŹż£©”£
Čē¹ūij»ģŗĻĀ±»ÆĮ×¹²ÓŠ3ÖÖ²»Ķ¬½į¹¹£Ø·Ö×ÓÖŠäåŌ×ÓĪ»ÖĆ²»ĶźČ«ĻąĶ¬µÄ½į¹¹£©£¬øĆ»ģŗĻĀ±»ÆĮ×µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ ”£
4£©Į×ėę»ÆŗĻĪļŗ¬ÓŠ3ÖÖŌŖĖŲ£¬ĒŅ·Ö×ÓÖŠŌ×Ó×ÜŹżŠ”ÓŚ20”£0.10mol PCl5ŗĶ0.10mol NH4ClĒ”ŗĆĶźČ«·“Ó¦£¬Éś³ÉĀČ»ÆĒāŗĶ0.030molĮ×ėę»ÆŗĻĪļ”£ĶĘĖćĮ×ėę»ÆŗĻĪļµÄĻą¶Ō·Ö×ÓÖŹĮæ£ØĢįŹ¾£ŗM>300£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĶ¼±ķŹ¾Ä³¹ĢĢ¬µ„ÖŹA¼°Ęä»ÆŗĻĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ£ØijŠ©²śĪļŗĶ·“Ó¦Ģõ¼žŅŃĀŌČ„£©”£»ÆŗĻĪļBŌŚ³£ĪĀ³£Ń¹ĻĀĪŖĘųĢ壬BŗĶCµÄĻą¶Ō·Ö×ÓÖŹĮæÖ®±ČĪŖ4£ŗ5£¬»ÆŗĻĪļDŹĒÖŲŅŖµÄ¹¤ŅµŌĮĻ”£
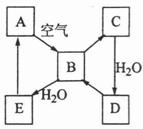
£Ø1£©Š“³öAŌŚ¼ÓČČĢõ¼žĻĀÓėH2·“Ó¦µÄ»Æѧ·½³ĢŹ½
£Ø2£©Š“³öEÓėAµÄĒā»ÆĪļ·“Ӧɜ³ÉAµÄ»Æѧ·½³ĢŹ½
£Ø3£©Š“³öŅ»øöÓÉDÉś³ÉBµÄ»Æѧ·½³ĢŹ½ ;
£Ø4£©½«5mL0.10mol”¤L-1µÄEČÜŅŗÓė10mL0.10mol”¤L-1µÄNaOHČÜŅŗ»ģŗĻ”£
¢ŁŠ“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½ ;
¢Ś·“Ó¦ŗóČÜŅŗµÄpH 7£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©£¬ĄķÓÉŹĒ ;
¢Ū¼ÓČČ·“Ó¦ŗóµÄČÜŅŗ£¬ĘäpH £ØĢī ”°Ōö“ó”±”¢”°²»±ä”±»ņ”°¼õŠ””±£©£¬ĄķÓÉŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹Ų¹¤ŅµÉś²śµÄŠšŹöÕżČ·µÄŹĒ
A.ŗĻ³É°±Éś²ś¹ż³ĢÖŠ½«NH3Ņŗ»Æ·ÖĄė£¬æɼÓæģÕż·“Ó¦ĖŁĀŹ£¬ĢįøßN2”¢H2µÄ×Ŗ»ÆĀŹ
B.ĮņĖį¹¤ŅµÖŠ£¬ŌŚ½Ó“„ŹŅ°²×°ČČ½»»»Ę÷ŹĒĪŖĮĖĄūÓĆSO3×Ŗ»ÆĪŖH2SO4Ź±·Å³öµÄČČĮæ
C.µē½ā±„ŗĶŹ³ŃĪĖ®ÖĘÉÕ¼ī²ÉÓĆĄė×Ó½»»»Ä¤·Ø£¬æÉ·ĄÖ¹Ņõ¼«ŹŅÉś²śµÄCl2½ųČėŃō¼«ŹŅ
D.µē½ā¾«Į¶ĶŹ±£¬Ķ¬Ņ»Ź±¼äÄŚŃō¼«ČܽāĶµÄÖŹĮæ±ČŅõ¼«Īö³öĶµÄÖŹĮ抔
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
“æ¼īŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£ÄæĒ°ÖĘ¼ī¹¤ŅµÖ÷ŅŖÓŠ”°°±¼ī·Ø”±ŗĶ”°ĮŖŗĻÖĘ¼ī·Ø”±Į½ÖÖ¹¤ŅÕ”£Ēė°“ŅŖĒó»Ų“šĪŹĢā£ŗ
£Ø1£©”°°±¼ī·Ø”±²śÉś“óĮæCaCl2·ĻĘśĪļ£¬ĒėŠ“³öøĆ¹¤ŅÕÖŠ²śÉśCaCl2µÄ»Æѧ·½³ĢŹ½£ŗ
 £»
£»
£Ø2£©Š“³ö”°ĮŖŗĻÖĘ¼ī·Ø”±ÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
£»
£»
£Ø3£©CO2ŹĒÖĘ¼ī¹¤ŅµµÄÖŲŅŖŌĮĻ£¬”°ĮŖŗĻÖĘ¼ī·Ø”±Óė”°°±¼ī·Ø”±ÖŠCO2µÄĄ“Ō“ÓŠŗĪ²»Ķ¬£æ
 £»
£»
£Ø4£©ĀĢÉ«»ÆѧµÄÖŲŅŖŌŌņÖ®Ņ»ŹĒĢįøß·“Ó¦µÄŌ×ÓĄūÓĆĀŹ”£øł¾Ż”°ĮŖŗĻÖĘ¼ī·Ø”±×Ü·“Ó¦£¬ĮŠ³ö¼ĘĖćŌ×ÓĄūÓĆĀŹµÄ±ķ“ļŹ½£ŗ
Ō×ÓĄūÓĆĀŹ£Ø%£©= ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŹµŃéŹŅĄļæÉÓĆĻĀĶ¼ĖłŹ¾×°ÖĆÖĘČ”ĀČĖį¼Ų”¢“ĪĀČĖįÄĘŗĶĢ½¾æĀČĖ®µÄŠŌÖŹ”£
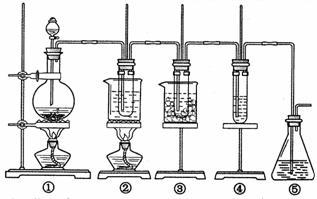
Ķ¼ÖŠ£ŗ¢ŁĪŖĀČĘų·¢Éś×°ÖĆ£»¢ŚµÄŹŌ¹ÜĄļŹ¢ÓŠ15mL30%KOH ČÜŅŗ.²¢ÖĆÓŚĖ®Ō”ÖŠ£» ¢Ū µÄŹŌ¹ÜĄļŹ¢ÓŠ15mL 8 % NaOH ČÜŅŗ.²¢ÖĆÓŚ±łĖ®Ō”ÖŠ£» ¢Ü µÄŹŌ¹ÜĄļ¼ÓÓŠ×ĻÉ«ŹÆČļŹŌŅŗ£» ¢Ż ĪŖĪ²ĘųĪüŹÕ×°ÖĆ”£
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©ÖĘČ”ĀČĘųŹ±£¬ŌŚÉÕĘæĄļ¼ÓČĖŅ»¶ØĮæµÄ¶žŃõ»ÆĆĢ.Ķعż________________£ØĢīŠ“ŅĒĘ÷Ćū³Ę£©ĻņÉÕĘæÖŠ¼ÓČĖŹŹĮæµÄÅØŃĪĖį”£ŹµŃ鏱ĪŖĮĖ³żČ„ĀČĘųÖŠµÄĀČ»ÆĒāĘųĢ壬æÉŌŚ¢Ł Óė¢Ś Ö®¼ä°²×°Ź¢ÓŠ___________£ØĢīŠ“ĻĀĮŠ±ąŗÅ×ÖÄø£©µÄ¾»»Æ×°ÖĆ”£
A.¼īŹÆ»Ņ B.±„ŗĶŹ³ŃĪĖ® C.ÅØĮņĖį D.±„ŗĶĢ¼ĖįĒāÄĘČÜŅŗ
£Ø2£©±Č½ĻÖĘČ”ĀČĖį¼ŲŗĶ“ĪĀČĖįÄʵÄĢõ¼ž£¬¶žÕߵIJīŅģŹĒ£ŗ_______________________________
·“Ó¦Ķź±Ļ¾ĄäČ“ŗ󣬢ŚµÄŹŌ¹ÜÖŠÓŠ“óĮ澧ĢåĪö³ö”£ĻĀĶ¼ÖŠ·ūŗĻøĆ¾§ĢåČܽā¶ČĒśĻߵďĒ________£ØĢīŠ“±ąŗÅ×ÖÄø£©£»“Ó¢ŚµÄŹŌ¹ÜÖŠ·ÖĄė³öøĆ¾§ĢåµÄ·½·ØŹĒ________________(ĢīŠ“ŹµŃé²Ł×÷Ćū³Ę£©

(3)±¾ŹµŃéÖŠÖĘČ”“ĪĀČĖįÄʵĥė×Ó·½³ĢŹ½ŹĒ£ŗ_____________________________
(4)ŹµŃéÖŠæɹŪ²ģµ½¢ÜµÄŹŌ¹ÜĄļČÜŅŗµÄŃÕÉ«·¢ÉśĮĖČēĻĀ±ä»Æ£¬ĒėĢīŠ“ĻĀ±ķÖŠµÄæÕ°×£ŗ
| ŹµŃéĻÖĻó | ŌŅņ |
| ČÜŅŗ×ī³õ“Ó×ĻÉ«Öš½„±äĪŖ____É« | ĀČĘųÓėĖ®·“Ӧɜ³ÉµÄH+Ź¹ŹÆČļ±äÉ« |
| ĖęŗóČÜŅŗÖš½„±äĪŖĪŽÉ« | _______________________________________________ |
| Č»ŗóČÜŅŗ“ÓĪŽÉ«Öš½„±äĪŖ____É« | _______________________________________________ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
U”¢V”¢W”¢X”¢Y”¢ZŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄĮłÖÖ³£¼ūŌŖĖŲ”£YµÄµ„ÖŹŌŚW2ÖŠČ¼ÉյIJśĪļæÉŹ¹Ę·ŗģČÜŅŗĶŹÉ«”£ZŗĶWŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļZ3W4¾ßÓŠ“ÅŠŌ”£UµÄµ„ÖŹŌŚW2ÖŠČ¼ÉÕæÉÉś³ÉUWŗĶUW2Į½ÖÖĘųĢ唣X µÄµ„ÖŹŹĒŅ»ÖÖ½šŹō£¬øĆ½šŹōŌŚUW2ÖŠ¾ēĮŅČ¼ÉÕÉś³ÉŗŚ”¢°×Į½ÖÖ¹ĢĢ唣
µÄµ„ÖŹŹĒŅ»ÖÖ½šŹō£¬øĆ½šŹōŌŚUW2ÖŠ¾ēĮŅČ¼ÉÕÉś³ÉŗŚ”¢°×Į½ÖÖ¹ĢĢ唣
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©VµÄµ„ÖŹ·Ö×ӵĽį¹¹Ź½ĪŖ £»XWµÄµē×ÓŹ½ĪŖ £»ZŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ ”£
£Ø2£©UŌŖĖŲŠĪ³ÉµÄĶ¬ĖŲŅģŠĪĢåµÄ¾§ĢåĄąŠĶæÉÄÜŹĒ£ØĢīŠņŗÅ£© ”£
¢ŁŌ×Ó¾§Ģå ¢ŚĄė×Ó¾§Ģå ¢Ū·Ö×Ó¾§Ģå ¢Ü½šŹō¾§Ģå
£Ø3£©U”¢V”¢WŠĪ³ÉµÄ10µē×ÓĒā»ÆĪļÖŠ£¬U”¢VµÄĒā»ÆĪļ·Šµć½ĻµĶµÄŹĒ£ØŠ“»ÆѧŹ½£©
£»V”¢WµÄĒā»ÆĪļ·Ö×Ó½įŗĻH+ÄÜĮ¦½ĻĒæµÄŹĒ£ØŠ“»ÆѧŹ½£© £»ÓĆŅ»øöĄė×Ó·½³ĢŹ½¼ÓŅŌÖ¤Ć÷ ”£
£Ø4£©YW2ĘųĢåĶØČėBaCl2ŗĶHNO3µÄ»ģŗĻČÜŅŗ£¬Éś³É°×É«³ĮµķŗĶĪŽÉ«ĘųĢåVW£¬ÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £¬ÓÉ“ĖæÉÖŖVWŗĶYW2»¹ŌŠŌ½ĻĒæµÄŹĒ£ØŠ“»ÆѧŹ½£© ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĶ¼ŹĒ²æ·Ö¶ĢÖÜĘŚŌŖĖŲµÄµ„ÖŹ¼°Ęä»ÆŗĻĪļµÄ×Ŗ»Æ¹ŲĻµĶ¼£ØÓŠ¹Ų·“Ó¦µÄĢõ¼ž¼°Éś³ÉµÄH2OŅŃĀŌČ„£©£¬ŅŃÖŖ£ŗ(a)A”¢B”¢C”¢DŹĒ·Ē½šŹōµ„ÖŹ£¬ĘäÖŠB”¢C”¢DŌŚ³£ĪĀ³£Ń¹ĻĀŹĒĘųĢ唣(b)·“Ó¦¢Ł”¢¢ŚŹĒ»Æ¹¤Éś²śÖŠµÄÖŲŅŖ·“Ó¦”£(c)»ÆŗĻĪļEŹĒŠĪ³ÉĖįÓźµÄĪŪČ¾ĪļÖ®Ņ»£¬»ÆŗĻĪļKŹĒ³£ÓƵĵŖ·Ź”£(d)»ÆŗĻĪļL¾ßÓŠĘư׊Ō£¬æÉÓÉCl2ÓėNaOHČÜŅŗ·“Ó¦¶ųÖʵƔ£(e)»ÆŗĻĪļJÓÉĮ½ÖÖŌŖĖŲ×é³É£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ32”£
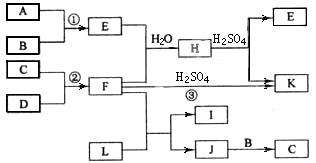
Ēė°“ŅŖĒóĢīæÕ£ŗ
¢Å·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½__________________________”£
¢ĘCµÄ½į¹¹Ź½______________£»HµÄ»ÆѧŹ½______________”£
¢ĒLµÄČÜŅŗÓė»ÆŗĻĪļE·“Ó¦µÄĄė×Ó·½³ĢŹ½__________________________”£
¢Č»ÆŗĻĪļJµÄ»ÆѧŹ½______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·“Ó¦ÖŠĢśŌŖĖŲ±»»¹ŌµÄŹĒ£Ø””””£©
| ”” | A£® | 2Fe£ØOH£©3 | B£® | Fe+CuSO4=Cu+FeSO4 |
| ”” | C£® | Fe£ØOH£©3+3HCl=FeCl3+3H2O | D£® | 2Fe2O3+3C |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com