
| |||||||||||||||||||
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2007?�Ͳ���ģ������������������һ�ֳ��õĽ������洦������������ʹ���ı�������һ�����ܵ�����Ĥ��������Ĥ������ϡ���ᣮij��ѧ�о�С����ʵ�����а����в���ģ����������̣���д���пհף�
��2007?�Ͳ���ģ������������������һ�ֳ��õĽ������洦������������ʹ���ı�������һ�����ܵ�����Ĥ��������Ĥ������ϡ���ᣮij��ѧ�о�С����ʵ�����а����в���ģ����������̣���д���пհף��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �Խ���������д������γ�һ�����ܵ������ﱣ��Ĥ���Ƿdz���Ч��һ�ֽ�������������
�Խ���������д������γ�һ�����ܵ������ﱣ��Ĥ���Ƿdz���Ч��һ�ֽ��������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
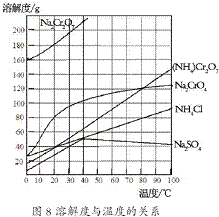 ��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�
��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�2- 3 |
2- 6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣�����������һ�ְ�ȫ���������ͨ�����в���CaO���Ҵ����������ȵĽᾧˮ��Ϊ����ij����������Ʒ����ɣ�����������ʵ�顣
�� ��ȡ0.270 g��Ʒ������ʹ֮��ȫ�ֽ⣬����CaO��O2��H2O���õ���O2�ڱ�״�������Ϊ33.6 mL��
�� ��ȡ0.120 g��Ʒ������ϡ���ᣬ������У�ʹ���ɵ�H2O2��ȫ�ֽ⡣Ȼ����Һ�е�Ca2+ ��ȫת��ΪCaC2O4������������ϴ�Ӻ����������ȵ�ϡ���ᣬ��0.0200 mol��L���� KMnO4��Һ�ζ�������ȥ31.0 mL KMnO4��Һ����ѧ����ʽ���£�
5CaC2O4��2KMnO4��8H2SO4 �� K2SO4��2MnSO4��5CaSO4��10CO2����8H2O
(1) д��CaO2���ȷֽ�Ļ�ѧ����ʽ��
(2) ������Ʒ��CaO2������������
(3) ������Ʒ��CaO2��x H2O��xֵ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com