
����Ŀ���绯ѧ�����������ж�������Ҫ�����ú����塣
(1)ͼ1Ϊ��ɫ��Դ��������(CH3OCH3)ȼ�ϵ�����Ĺ���ԭ��ʾ��ͼ���õ�صĸ�����ӦʽΪ_________��

(2)�Ʊ����������ƣ�Na2S2O5���ɲ�������Ĥ��⼼����װ����ͼ2��ʾ������SO2������Һ�к���NaHSO3��Na2SO3�������ĵ缫��ӦʽΪ_______������____�ҵ�NaHSO3Ũ�����ӡ���������Һ���нᾧ��ˮ���ɵõ�Na2S2O5��
(3)�������ȣ�ClO2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ������������ش��������⣺
ʵ������NH4Cl�����ᡢNaClO2(�������ƣ�Ϊԭ�ϣ�ͨ�����¹����Ʊ�ClO2�����ʱ������Ӧ�Ļ�ѧ����ʽΪ________________����ҺX�д������ڵ���������________________��

(4)�ⶨ�������ClO2�ĺ�����
��.����ƿ�м��������ĵ⻯�أ���50 mLˮ�ܽ���ټ���3 mLϡ���ᡣ��һ�����Ļ������ͨ������Һ�г�����ա�
��.��0.1000 mol��L-1��������Ʊ���Һ�ζ���ƿ�е���Һ��I2+2S2O32-��2I��+S4O62-)���Ե�����ҺΪָʾ����ʾ�յ�ʱ����ȥ20.00 mL�����������Һ��
����ƿ��ClO2��⻯�ط�Ӧ�����ӷ���ʽΪ__________________��
�ڲ�û������ClO2������Ϊ______��
�۲ⶨ�������ClO2�ĺ����IJ����п���ʹ�ⶨ���ƫ�͵���____(����ĸ)��
a.�ζ���δ��ϴ��ֱ��ע����������Ʊ�Һ
b.�ζ��ܶ�ȡ��Һ���ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
c.��ƿ������ˮϴ����û�и���
���𰸡�CH3OCH3-12e-+3H2O=2CO2+12H+ 2H2O��4e��=4H++O2����4OH-��4e- =O2��+2H2O a NH4Cl��2HCl![]() 3H2����NCl3 Cl-��OH- 2ClO2��10I����8H����2Cl����5I2��4H2O 0.0270g����0.027g�� b
3H2����NCl3 Cl-��OH- 2ClO2��10I����8H����2Cl����5I2��4H2O 0.0270g����0.027g�� b
��������
��1�������϶�����ʧȥ�������ɶ�����̼�������������Һ��
��2����ͼ��֪�����Ϊ����������������ʧȥ���ӣ��������������ƶ���
��3�����������̿�֪�Ȼ����������Һ�н��е�⣬����������������������NCl3����ⷽ��ʽΪNH4Cl��2HCl![]() 3H2����NCl3����NCl3��Һ�м���NaClO2��������ClO2��NH3��X��X�к�Cl-��OH-��
3H2����NCl3����NCl3��Һ�м���NaClO2��������ClO2��NH3��X��X�к�Cl-��OH-��
��4����ClO2��⻯�ط���������ԭ�ֱ�������������ⵥ�ʣ�����������ԭ��Ӧ�Ĺ�����ƽ���ɣ�
�ڵ����������������2ClO2+10I-+8H+�T2Cl-+5I2+4H2O��I2+2S2O32-�T2I-+S4O62-�����2ClO2��5I2��10Na2S2O3���㣻
�����ı�Һ�����ƫСʱ���ⶨ���ƫ�͡�
(1)�õ���У����������ѷ���ʧ���ӵ�������Ӧ���为����ӦʽΪCH3OCH3-12e-+3H2O=2CO2+12H+��
�ʴ�Ϊ��CH3OCH3-12e-+3H2O=2CO2+12H+��
(2)��������Ϊϡ������Һ���������Һ�����ԣ�������������������Ӧ������ӦΪH2O�ŵ磬����O2��H+����缫��ӦΪ��2H2O��4e��=4H++O2����4OH-��4e- =O2��+2H2O����Һ�зָ������ҵ�ĤΪ�����ӽ���Ĥ���������������ƶ���H+ͨ�������ӽ���Ĥ����a�ң���a�ҵ�SO32-������Ӧ��H++SO32-=HSO3-����˵���a�ҵ�NaHSO3Ũ�����ӣ�NaHSO3��������Һ���ᾧ��ˮ�Ƶ�Na2S2O5���ù��̵Ļ�ѧ����ʽ2NaHSO3=Na2S2O5+H2O��
�ʴ�Ϊ��2H2O��4e��=4H++O2����4OH-��4e- =O2��+2H2O��a��
(3)�����������������������ԭ��Ӧ���ɿ�֪�����ʱ������Ӧ�Ļ�ѧ����ʽΪNH4Cl��2HCl![]() 3H2����NCl3��NCl3��NaClO2��Һ��Ӧ��NCl3+6NaClO2+3H2O=6ClO2��+NH3��+3NaCl+3NaOH��������ҺX�д������ڵ���������Cl-��OH-��
3H2����NCl3��NCl3��NaClO2��Һ��Ӧ��NCl3+6NaClO2+3H2O=6ClO2��+NH3��+3NaCl+3NaOH��������ҺX�д������ڵ���������Cl-��OH-��
�ʴ�Ϊ��NH4Cl��2HCl![]() 3H2����NCl3�� Cl-��OH-��
3H2����NCl3�� Cl-��OH-��
(4)��ClO2��⻯�ط���������ԭ�ֱ�������������ⵥ�ʣ���ϵ���ת�����غ�͵���غ㡢ԭ���غ��֪���÷�Ӧ�����ӷ���ʽΪ��2ClO2+10I-+8H+�T2Cl-+5I2+4H2O��
�ڵζ����յ����������Һ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٸı䣬
����Na2S2O3���ʵ���Ϊ0.02L��0.1mol/L=0.002mol�������2ClO2��5I2��10Na2S2O3��n(ClO2)=![]() n(Na2S2O3)����n(ClO2)=0.0004 mol������m(ClO2)=0.004mol��67.5g/mol=0.0270g����0.027g����
n(Na2S2O3)����n(ClO2)=0.0004 mol������m(ClO2)=0.004mol��67.5g/mol=0.0270g����0.027g����
��a. �ζ���δ��ϴ��ֱ��ע����������Ʊ�Һ�����ı�Һ�����ƫ�ⶨ���ƫ�ߣ��ʲ�ѡ��
b. �ζ��ܶ�ȡ��Һ���ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������ı�Һ�����ƫСʱ���ⶨ���ƫ�ͣ���ѡ��
c. ��ƿ������ˮϴ����û�и����ʵ����Ӱ�죬�ʲ�ѡ��
�ʴ�Ϊ��b��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ȣ�C1O2��������ˮ����һ�ִ���ˮ�ĸ�Ч��ȫ��������ʵ������NH4C1�����ᡢNaClO2Ϊԭ���Ʊ�C1O2���������¡���֪NCl3������95��ͻᷢ����ը�Էֽ⣬����ˮ���ֽ⣬�ڿ������ӷ�������˵��������ǣ� ��

A.��ȥC1O2�е�NH3��ѡ�ñ���ʳ��ˮ
B.���ʱ��������Ӧ�Ļ�ѧ����ʽΪNH4C1+2HCl![]() 3H2��+NCl3
3H2��+NCl3
C.X��Һ�д������ڵ�������Na+��C1-��H+
D.Ϊ��֤ʵ��İ�ȫ���ڵ��ʱҪ���ƺ÷�Ӧ�¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڻ�ѧ��Ӧ�е������仯������˵���в���ȷ����( )
A. ȼ�շ�Ӧ���Ƿ��ȷ�Ӧ
B. ���ڿ��淴Ӧ��aA(g)+bB(g)![]() bC(g)+dD(g)���������Ӧ���ȣ��淴Ӧһ������
bC(g)+dD(g)���������Ӧ���ȣ��淴Ӧһ������
C. ����ȼ������ˮ��һ�����ȵĻ�ѧ��Ӧ��˵��1 mol H2����������1 mol H2O������
D. ֻ�з��ȵ�������ԭ��Ӧ�ſ������Ϊԭ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС�����������װ�ý���ʵ�飬�ڡ�������Һ���������������������±���
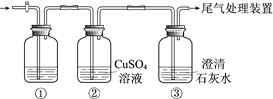
ʵ�� | ���� | ���� |
�� | ��ʢ��Na2S��Һ�Ģ��г���ͨ��CO2������ | ���в�����ɫ��������Һ��pH���ͣ� ���в�����ɫ���ǣ��û�������ð���� |
�� | ��ʢ��NaHCO3��Һ�Ģ��г���ͨ��H2S���������� | ����ͬʵ��� |
���ϣ�CaS��ˮ��ȫˮ��
������ʵ��ó��Ľ�������ȷ����
A.���а�ɫ������CaCO3
B.������ҺpH���͵�ԭ���ǣ�H2S+Cu2+ == CuS��+2H+
C.ʵ�����з����ķ�Ӧ�ǣ�CO2+H2O+ S2== CO32+ H2S
D.��ʵ���͢��ܱȽ�H2CO3��H2S���Ե�ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�������μӵ�Na2X��Һ�У������Һ��pOH[pOH=��lgc(OH-)]������Ũ�ȱ仯�Ĺ�ϵ��ͼ��ʾ��

����������ȷ����
A. ����N��ʾpOH�� ���ߵı仯��ϵ
���ߵı仯��ϵ
B. NaHX��Һ��c(X2-)>c(H2X)
C. �������Һ������ʱ��c(Na+)=c(HX-)+2c(X2-)
D. �����£�Na2X�ĵ�һ��ˮ�ⳣ��Kh1=1.0��10-4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£��� 100 mL0.01 mol��L-1HA ��Һ����μ���0.02 mol��L-1MOH ��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���(����仯���Բ��ƣ����ش���������:

��1����ͼ����Ϣ��֪HAΪ ��(�ǿ������������������ ��
��2��������һ��Ũ�ȵ�MAϡ��Һ��pH = a����a_________7(� > ������< ����=�����������ӷ���ʽ��ʾ��ԭ����_____________________����ʱ����Һ����ˮ�������c(OH-��= ��
��3����д��K������Ӧ����Һ������Ũ�ȵĴ�С��ϵ��____________________��
��4��K���Ӧ����Һ�У�c(M+�� +c(MOH��__________2c (A-��(�>����<����=����������ʱ��Һ�� pH = 10���� c(MOH�� +c(OH-�� =_____________mol�� L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
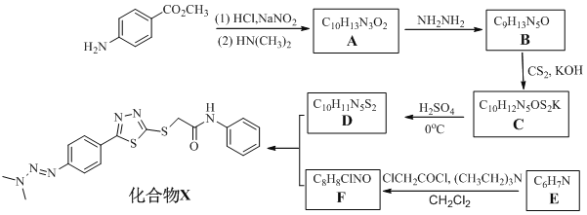
����Ŀ��������֣�ݴ�ѧҩѧԺij�о�С��ϳɳ����п��������ԵĻ�����X����ϳ�·������ͼ��ʾ��
��֪��
a.R-SH+R��Cl��RS-R��
b 
c 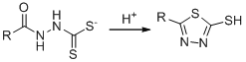
��ش�
��1��������F�Ľṹ��ʽ_________________���Ʋ�E��ClCH2COCl��Ӧ�õ�������F�Ĺ����У� ���ȼ���(CH2Cl2)�Ƿ�Ӧ���ܼ�����(CH3CH2)3N��������_________________��
��2������˵����ȷ����___________________��
A. ������D�����Ʒ�Ӧ
B. ��������B��F��������X�ķ�Ӧ��Ϊȡ����Ӧ
C. 1 mol ������B������5 mol H2�����ӳɷ�Ӧ
D. ������X����ʽΪC18H18N6OS2
��3��A��B�Ļ�ѧ����ʽΪ__________________________________��
��4��д��ͬʱ��������������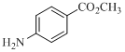 ��ͬ���칹��Ľṹ��ʽ��______________��
��ͬ���칹��Ľṹ��ʽ��______________��
�ٺ��������ʾ�����к���һ������1H-NMR��ͼ��ʾ�����������ֻ�ѧ�������⣻
�ڷ�����ˮ�⣬���ܷ���������Ӧ
��5������Լױ����״�Ϊԭ�Ϻϳɻ�����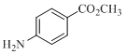 ��������ͼ��ʾ�����Լ���ѡ��________________________________��
��������ͼ��ʾ�����Լ���ѡ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ�ÿ��Խ���������CO2ת��Ϊȼ������CO.����˵���У���ȷ����( )
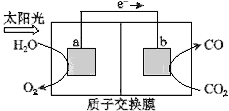
A.��װ�ù���ʱ��H����b������a�����ƶ�
B.��װ����ÿ����1 mol CO��ͬʱ����1 mol O2
C.�缫a���淢����ԭ��Ӧ
D.�ù����ǽ�̫����ת��Ϊ��ѧ�ܵĹ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ������ȷ����
A. NaHCO3��ˮ�⣺HCO3����H2O![]() H3O����CO32��
H3O����CO32��
B. ����ĵ��룺CH3COOH��CH3COO����H��
C. H2��ȼ����Ϊ285.8 kJ/mol�����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪ��H2(g)��1/2O2(g)��H2O(g) ��H����285.8 kJ/mol
D. ̼��Ƶ��ܽ�ƽ�⣺CaCO3(s)![]() Ca2��(aq)��CO32��(aq)
Ca2��(aq)��CO32��(aq)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com