
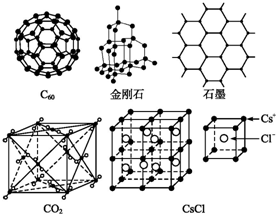
���� ��1��ͬ��Ԫ�صIJ�ͬ���ʻ���ͬ�������壻
��2�����ݾ��幹�����жϾ������ͣ�
��3��ÿ����ԭ�Ӻ���2��������
��4�����þ�̯�����㣻
��5���ɱ��Ƿ��Ӿ��壬CO2����λ��������Ķ���������ϣ��Զ����ϵ�CO2����Ϊ���������������CO2���ӷֲ�����ö���������12����������ϣ�
��6������CsCl����ľ���������
��7�����þ�̯��ȷ�����������к��е����ӣ����ݦ�V=$\frac{M}{{N}_{A}}$������Է���������
��8��NaCl����Ϊ���������ܶѻ������ı߳�Ϊ��2a+2b��pm�������Na+��Cl-��ռ����;���������Ӷ�������ռ������ʣ�
��� �⣺��1��ͬ��Ԫ�صIJ�ͬ���ʻ���ͬ�������壬C60�����ʯ��ʯī��̼Ԫ�صIJ�ͬ���ʣ�����ͬ�������壻
�ʴ�Ϊ��B��
��2��C60�й������Ƿ��ӣ��������ڷ��Ӿ��壻
��C60�����к���x������κ�y�������Σ�����ŷ���������������ÿ��������������⣬��ÿ�������������������㹲�У�ÿ�����㵥�����������1.5����60�����㹲�������Ϊ60��1.5=90�������ˣ�����ŷ��������д����60+x+y-90=2 ��
ÿ�������������湲�õģ����ԣ�һ������ε���ռ�е������Ϊ2.5����һ�������ε���ռ�е������Ϊ3������������غ�2.5x+3y=90 �������٢ڿ��Խ�ã�x=12��y=20��ͨ�����Ϸ���֪����ε�̼�γɹ���˫��������ε�̼�γɹ��۵���������һ����������30��̼̼˫����
�ʴ�Ϊ�����ӣ�30��
��3��ÿ����ԭ�Ӻ���2����������1mol�辧���к��й�赥������ĿԼ��2NA����
�ʴ�Ϊ��2��
��4��ʯī��״�ṹ�У�ÿ��̼ԭ�ӱ������������ι��ã�����ƽ��ÿ����������ռ�е�̼ԭ����=6��$\frac{1}{3}$=2��
�ʴ�Ϊ��2��
��5���ɱ��Ƿ��Ӿ��壬CO2����λ��������Ķ���������ϣ��Զ����ϵ�CO2����Ϊ�����������������CO2���ӷֲ�����ö���������12����������ϣ������з�����Ŀ=8��$\frac{1}{8}$=4��
�ʴ�Ϊ��12��4��
��6��ÿ��Cs+��Χ��������Ҿ�����ȵ�Cs+����$3��8��\frac{1}{4}$=6����
ÿ��Cs+��Χ��������Ҵν���Cs+Ϊ�����ϵ�CSԭ�ӣ�Ŀ=$3��8��\frac{1}{2}$=12������Ϊ$\sqrt{2}a$��
��������ҵ�������Cs+Ϊ�����ϵģ���ĿΪ1��8=8������Ϊ$\sqrt{3}a$��
ÿ��Cs+��Χ�����ҵȾ��Cl-��Ŀ=1��8=8��
�ʴ�Ϊ��6��12��$\sqrt{2}a$��8��$\sqrt{3}a$��8��
��7�����������к�1�������ӣ�1��Cs+�����ݦ�V=$\frac{M}{{N}_{A}}$֪����=$\frac{M}{{N}_{A}{a}^{3}}$=$\frac{168.5}{{N}_{A}{a}^{3}}$��
�ʴ�Ϊ��$\frac{168.5}{{N}_{A}{a}^{3}}$��
��8�������й�����1��Cs+��1��Cl-�����Ϊ��$\frac{4}{3}$�У�r13+r23����1�������ı߳�Ϊ2��$\frac{1}{\sqrt{3}}$��r1+r2���������Ϊ[$\frac{1}{\sqrt{3}}$��2r1+2r2��]3���Ȼ�菉��������ӵĿռ�������Ϊ��$\frac{\frac{4}{3}�У�{{r}_{1}}^{3}+{{r}_{2}}^{3}��}{[\frac{2}{\sqrt{3}}��{r}_{1}+{r}_{2}��]^{3}}$=$\frac{\sqrt{3}�У�{{r}_{1}}^{3}+{{r}_{2}}^{3}��}{2��{r}_{1}+{r}_{2}��^{3}}$��
�ʴ�Ϊ��$\frac{\sqrt{3}�У�{{r}_{1}}^{3}+{{r}_{2}}^{3}��}{2��{r}_{1}+{r}_{2}��^{3}}$��
���� ���⿼���˾����Ľṹ�������ڿ��龧���ṹ�ķ����ͼ��㣬ע�����þ�̯�����㾧���и���ԭ�Ӹ�������Ŀ�Ѷȴ���ؼ�����ϸ�۲쾧���ṹͼ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ�������£�0.2 mol SO2������O2��ַ�Ӧ������SO3������Ϊ0.2NA | |
| B�� | 0.1 mol NH2-�к��еĵ�����Ϊ0.9NA | |
| C�� | 3.4 g H2O2�к��еĹ��õ��Ӷ���Ϊ0.1NA | |
| D�� | ���³�ѹ�£�16 g O2��O3������庬�е���ԭ����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Al�������Ȼ����Һ���ҷų����� | |
| B�� | Mg���ڿ�����ȼ�տ��������ֲ��� | |
| C�� | MgO�����ӻ�������Թ�ҵ���õ��������̬�ķ�����ȡMg | |
| D�� | ��Al2��SO4��3��Һ�еμӹ�����ˮ���ɵð�ɫ�������������NaHSO4��Һ������ʧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ͭ��Һ��H2S��Ӧ�����ӷ���ʽ��Cu2++S2-=CuS�� | |
| B�� | ��������Һ�����Ũ��ˮ��Ӧ�����ӷ���ʽ��Fe3++3NH3•H2O=Fe��OH��3��+3NH4+ | |
| C�� | H2��H2S��SO2��CO2�������嶼����Ũ������� | |
| D�� | SO2��SO3�������ͨ��Ba��NO3��2��Һ�ɵõ�BaSO3��BaSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
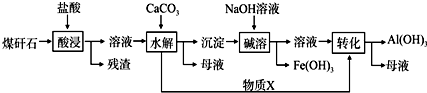
 ���ù�������У�����X���뷴Ӧ�����ӷ���ʽ�ǣ�AlO2-+CO2+2H2O=Al��OH��3+HCO3-��
���ù�������У�����X���뷴Ӧ�����ӷ���ʽ�ǣ�AlO2-+CO2+2H2O=Al��OH��3+HCO3-���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
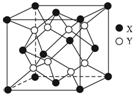 ij���Ӿ���ľ����ṹ��ͼ��ʾ��
ij���Ӿ���ľ����ṹ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��100mL1.0mol•L-1��NaOH��Һ��ȡ��10mL����Һ������ҺŨ����Ϊ1.0mol•L-1 | |
| B�� | ����ƾ���ˮ���Բ��÷�Һ�ķ��� | |
| C�� | ������NaCl��Һʱ��������������Һ��ʱ��ֹͣ���ȣ��������ȼ������� | |
| D�� | ��ʢ�е�ˮ���Թ��м���������CCl4������²���ɫ��Ϊ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ��
��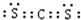 ���ṹʽ�ǣ�S=C=S��
���ṹʽ�ǣ�S=C=S���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����13.5% | B�� | ����12.5% | C�� | С��12.5% | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com