
25��ʱ����20 mL 0.1 mol?L��1 NaOH��Һ����μ���0.2 mol?L��1 ������Һ��������ͼ��ʾ���й�����Ũ�ȹ�ϵ�ıȽ�������ȷ���ǣ��� ��
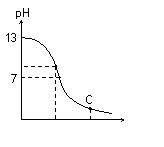
A����A�㣺c(Na��)��c(OH��)��c(CH3COO��)��c(H��)
B����B�㣺c(OH��)��c(H��)��c(Na��)��c(CH3COO��)
C����C�㣺c(CH3COO��)��c(Na��)��c(H��)��c(OH��)
D����C�㣺c(CH3COO��)��c(CH3COOH)��2c(Na��)

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
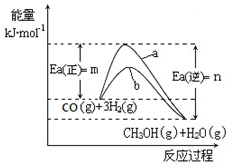
 CH3OH��g��+H2O��g����H=-��n-m��kJ?mol-1
CH3OH��g��+H2O��g����H=-��n-m��kJ?mol-1 CH3OH��g��+H2O��g����H=-��n-m��kJ?mol-1
CH3OH��g��+H2O��g����H=-��n-m��kJ?mol-1
| 16 |
| 3 |
| 16 |
| 3 |
 CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011
CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011 |
500 | 600 | 700 | 800 |
| 1.5 | 45 | 33 | 20 | 12 |
| 2.0 | 60 | 43 | 28 | 15 |
| 3.0 | 83 | 62 | 37 | 22 |
 ���¶����ߣ�Kֵ
���¶����ߣ�Kֵ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������� | A | B | C | D | ||
ƽ��ʱ
|
16 | 17 | ||||
| ƽ��ʱN2ת���� | 20% | �� | �� | �� | ||
| ƽ��ʱH2ת���� | 30% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡ��֦����2012��2013ѧ���һ��ѧ����ĩ���м�⻯ѧ���� ���ͣ�022
������
��HCl
��NaOH
��CH3COOH
��NH3��H2O
��CH3COONa
��NH4Cl
(1)����������ʵ���________(�����)����Һ�����Ե���________(�����)��
(2)25��ʱ��0.10 mol/LCH3COONa��ҺpH��11������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊ________��
(3)����pH�������HCl��CH3COOH�ֱ�ϡ��m����n������ϡ�ͺ�����Һ��PH����ȣ���m________n(����ڡ����ڡ�С�ڡ�)��
(4)25��ʱ����20 mL0.1 mol/L������Һ����VmL0.1 mol/LNaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����________��
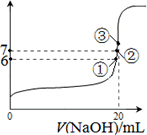
A��pH��3�Ĵ�����Һ��pH��11�Ĵ�������Һ�У�ˮ�ĵ���̶���ͬ
B���ٵ�ʱpH��6����ʱ��Һ�У�c(CH3COO��)��c(Na+)��9.9��10��7 mol/L
C���ڵ�ʱ����Һ�е�c(CH3COO��)��c(Na+)
D���۵�ʱV��20 mL����ʱ��Һ��c(CH3COO��)��c(Na+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��һ����6�֣� ��2��5g̼���ơ�̼�����ƺ��������ƹ���������ȫ�ܽ���ˮ���Ƴ�ϡ��Һ��Ȼ�������Һ����μ���1mol/L�����ᣬ�������������������CO2���������״������ϵ����ͼ��ʾ��
��1��д��OA����������Ӧ�����ӷ���ʽ
��2��������35mL����ʱ������������̼�����Ϊ mL����״����
��3��ԭ�������Na2CO3����������Ϊ ��
��������������Ҫ�ĵ��ʣ��Dz������Ļ�����Ʒ֮һ���α�����ܵĺϳɰ������й��������ǵ¹���ѧ�ҹ�����1905�귢���ģ���ϳ�ԭ��Ϊ��N2(g) + 3H2(g)![]() 2NH3(g)��
2NH3(g)��
��H���D92.4 kJ/mol������˻����1918���ŵ������ѧ�����Իش��������⣺
�� ���з������ʺ�ʵ������ȡ�������� ������ţ���
A������ʯ���е���Ũ��ˮ B������Ũ��ˮ
C��ֱ���������͵����ϳ� D�����Ȼ����Һ�е���Ũ����������Һ
�� �ϳɰ���ҵ�в�ȡ�����д�ʩ������������ԭ�����͵��� ������ţ���
A�����ýϸ�ѹǿ��20 M Pa��50 M Pa��
B������500��ĸ���
C��������ý������
D�������ɵİ�Һ������ʱ����ϵ�з��������δ��Ӧ��N2��H2ѭ�����ϳ�����
��3�� �����ֻ���ϢϵͳDIS������ͼ����ʾ�����ɴ����������ݲɼ����ͼ������ɣ����Բⶨ������ˮ��Ũ�ȡ�����ʽ�ζ���ȷ��ȡ0.5000 mol/L������Һ25.00 mL���ձ��У��Ը��ְ�ˮ���еζ����������Ļ����ʾ����Һ���������氱ˮ����仯����������ͼ����ʾ��
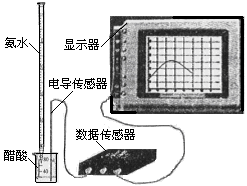
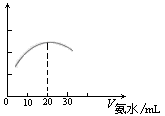
ͼ�� ͼ��
�� �õζ���ʢ��ˮǰ���ζ���Ҫ�� ��ϴ2��3�飬
�� �Լ�����ְ�ˮ��Ũ�ȣ� ��
�� ��������£��ᵼ��ʵ����c(NH3��H2O)ƫ�͵��� ��
A���ζ�����ʱ���Ӷ���
B����ȡ25.00 mL������Һʱ��δ����ʢ��Һ��ϴ�ζ���
C���ζ�ʱ����������ˮ�����ձ���
��4�� 1998��ϣ������ʿ��´�ѧ��Marnellos��Stoukides���ø����ӵ����Ե�SCY�մɣ��ܴ���H+����ʵ���˸��³�ѹ�¸�ת���ʵĵ绯ѧ�ϳɰ�����ʵ��װ������ͼ��
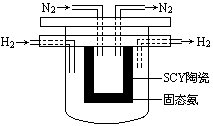
�����ĵ缫��ӦʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ѡ����
����ͼװ�ÿ����ڶ����ʵ�顣ͼ�мг̶ֹ�װ������ȥ�����п̶ȣ��������á�
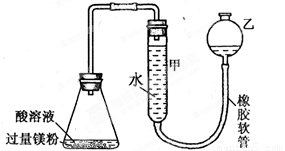
( 1 )װ�����п̶ȵļܿ�����______________���� (����������)����ͼ���Ӻ�װ�ú��װ�������Եķ�����______________________________
( 2 )ijʵ��С����þ�ۡ����ᡢ�������ʵ����֤������ͬ��ͬѹ�£�����������������ʵ�����ͬʱ����þ�۷�Ӧ���������������ͬ����Ӧ���ʲ�ͬ��װ������ͼ��ʾ��
�й�ʵ�����ݼ�¼���±���
|
����Һ |
����Һ |
���������mL |
��Ӧʱ�� |
|
|
(ʵ��A) |
(ʵ��B) |
(25�桢101 kPa) |
ʵ��A |
ʵ��B |
|
CH3COOH 0.1 mol��L 40.00mL |
HCl��Һ 0.1 mol��L 40.00mL |
5 |
t(a1)=155 s |
t(b1)=7 s |
|
10 |
t(a2)=310 s |
t(b2)=16 s |
||
|
15 |
t(a3)=465 s |
t(b3)=30 s |
||
|
20 |
t(a4)=665 s |
t(b4)=54 s |
||
|
���� |
���� |
���� |
��ش��������⣺
��ÿ��ʵ��������Ҫ�õ�����ƽ(�ܳ�1 mg) ��ȡþ��___________________g��
����ȴ��25����ڶ�ȡ�������ʱ������Ӧ��β�����__________________________��
�۷���ʵ�����ݣ�t(a1)ԶԶ����t(b1)��ԭ����__________________________��
��3����ͼʾװ��,ijͬѧ����˲ⶨ��п��Ƥ�Ʋ��ȵ�ʵ�鷽�������������ΪS cm2������Ϊm g�Ķ�п��Ƥ��6mol��L��1 NaOH��Һ��Ӧ���ش��������⣺����֪п���ܶ�Ϊ �� g/cm3��
�� д��Zn�Ʋ���NaOH��Һ��Ӧ�����ӷ���ʽ________________________________________
��Ϊ��߲ⶨ��ȷ�ԣ��轫��ƿ�ϵĵ���������Ϊ˫����������һ�ײ���______������������)
ʵ��ʱ������ƿ�м����п��Ƥ��Ʒ������˫�������ټ���NaOH��Һ��
�� ��֪ʵ��ǰ�����Һ�������ΪV mL��ʵ������������Ħ�����ΪVm mol��L��1�������п��Ƥ��п�Ʋ���Ϊ_________________________cm����д����ѧ����ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com