
 N��P��As��Ԫ�صĻ��������������о�����������Ҫ��;����ش��������⣺
N��P��As��Ԫ�صĻ��������������о�����������Ҫ��;����ش��������⣺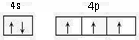 ������ͬ��������Ԫ�صĵ�һ�������ɴ�С��˳��ΪAs��Se��Ge��
������ͬ��������Ԫ�صĵ�һ�������ɴ�С��˳��ΪAs��Se��Ge������ ��1��N4���ӵĿռ乹����P4���ƣ�4��Nԭ���γ��������幹�ͣ�ÿ��Nԭ���γ�3��N-N����������1�Թ¶Ե��ӣ�
��2��AsԪ�ش��ڵ�������VA�壬�۵����Ų�ʽΪ4s24p3���������ԭ�������ع����۵����Ų�ͼ��Asԭ��4p���Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ�
��3��Ԫ�صĵ縺��ԽС���Թµ��Ӷ�����Խ����Խ�����γ���λ����
��4��PCl3��Pԭ���γ�3��P-Cl��������1�Թ¶Ե��ӣ���ռ�ṹ�����Σ����ȵ�����Ľṹ���ƣ�
ԭ�Ӱ뾶ԽС��ԭ��֮���γɵĹ��ۼ�Խ�̣�
��5�����þ�̯�����㾧���к���Gaԭ�ӡ�Asԭ����Ŀ������ȷ���仯ѧʽ��
Asԭ������Χ��4��Gaԭ���γ��������壬Asԭ���뾧������Gaԭ�����ߴ��ھ�����Խ����ϣ���Gaԭ����Asԭ��֮��ľ���Ϊ������Խ��߳��ȵ�$\frac{1}{4}$�������Ծ�������Խ���Ϊ�����ⳤ��$\sqrt{3}$������Ͼ����к���ԭ����Ŀ����ʾ�������������ٸ���m=��V���㾧���ⳤ��
��� �⣺��1��N4���ӵĿռ乹����P4���ƣ�4��Nԭ���γ��������幹�ͣ�ÿ��Nԭ���γ�3��N-N����������1�Թ¶Ե��ӣ��ӻ������ĿΪ4����Nԭ�Ӳ�ȡsp3�ӻ���ÿ�����Ϊ�������Σ���N-N���ļ���Ϊ60�㣬
�ʴ�Ϊ��sp3��60�㣻
��2��AsԪ�ش��ڵ�������VA�壬�۵����Ų�ʽΪ4s24p3���۵����Ų�ͼΪ�� ��
��
Asԭ��4p���Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ��ʵ�һ�����ܣ�As��Se��Ge��
�ʴ�Ϊ�� ��As��Se��Ge��
��As��Se��Ge��
��3��PԪ�صĵ縺�Ա�NԪ�ص�С��Pԭ�ӶԹµ��Ӷ������������������¶Ե����γ���λ����
�ʴ�Ϊ��PH3��
��4��PCl3��Pԭ���γ�3��P-Cl��������1�Թ¶Ե��ӣ���ռ�ṹ�����Σ����ȵ�����Ľṹ���ƣ�SCl3+�Ŀռ乹���������Σ�
S ԭ�Ӱ뾶С��Pԭ�Ӱ뾶���� S-Cl ��Ҫ�� P-Cl ����
�ʴ�Ϊ�������Σ�S ԭ�Ӱ뾶С��Pԭ�Ӱ뾶���� S-Cl ��Ҫ�� P-Cl ���̣�
��5�������к���Gaԭ����Ϊ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Asԭ����ĿΪ4���ʻ�ѧʽΪGaAs��
Asԭ������Χ��4��Gaԭ���γ��������壬Asԭ���뾧������Gaԭ�����ߴ��ھ�����Խ����ϣ���Gaԭ����Asԭ��֮��ľ���Ϊ������Խ��߳��ȵ�$\frac{1}{4}$�������Ծ�������Խ���Ϊ�����ⳤ��$\sqrt{3}$������������Ϊ4��$\frac{70+75}{{N}_{A}}$g�����ⳤ=$\root{3}{\frac{4��\frac{70+75}{{N}_{A}}}{��g•c{m}^{-3}}}$=$\root{3}{\frac{580}{��{N}_{A}}}$cm����a��b�ľ���Ϊ$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{580}{��{N}_{A}}}$cm=$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{580}{��{N}_{A}}}$��1010 pm��
�ʴ�Ϊ��GaAs��$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{580}{��{N}_{A}}}$��1010��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����ӻ���ʽ��ռ乹�͡������ܡ���λ���������������ȣ���5���о������Ϊ�״��㡢�ѵ㣬ע�����ù�ԭ�ӵ�ƽ�潫�����ĵȷ�ȷ��ab�����뾧����Խ��߳��ȹ�ϵ����ѧ���߱�һ���Ŀռ���������ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��

| A�� | ������ | B�� | �ƾ� | C�� | Ũ���� | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
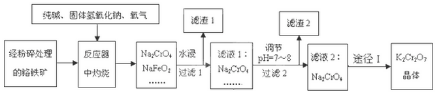
�鿴�𰸺ͽ���>>
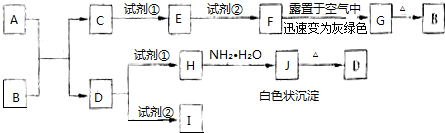
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �κξ����У������������Ӿ�һ���������� | |
| B�� | ԭ�Ӿ�����ֻ���й��ۼ� | |
| C�� | ԭ�Ӿ�����۵�һ���Ƚ�������ĸ� | |
| D�� | ���Ӿ�����ֻ���з��»��� |
�鿴�𰸺ͽ���>>
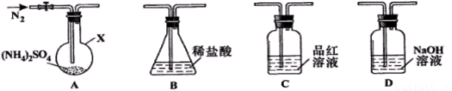
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
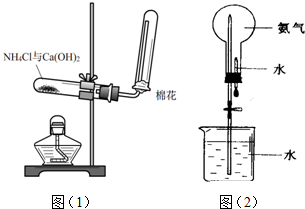
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
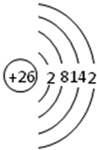
| A�� | X���⻯���γɵľ����д��ڵ���ǿ�������Ƿ��Ӽ������� | |
| B�� | R����̬�⻯������ˮ�����Ӽ����� | |
| C�� | W����̬�⻯�������ӻ����� | |
| D�� | Y��Z��������������Ӧ��ˮ���������Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com