
����7��)ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺
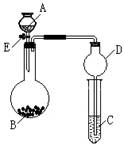
(1����AΪŨ��ˮ��BΪ�����ƣ�C��ʢ��AlCl3��Һ����������E���㹻��ʱ��۲쵽C�е�����Ϊ ��Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ ��
(2����AΪ30%��H2O2��Һ��BΪ�������̣�C��ʢ���ữ����FeCl2��Һ����������E��C�е�����Ϊ ��Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ ��
(3)��������װ�û�������֤SO2�Ļ�ѧ����, AΪ��Ũ���ᣬBΪ�������ƣ���C��ʢ��
��Һ����֤SO2��������; C��ʢ�� ��Һ����֤�仹ԭ��; ��C��ʢ�� ��Һ����֤��Ư���ԡ�
1���а�ɫ�������ɣ�CaO+NH3��H2O==Ca��OH��2+ NH3��
(2����ɫ��dz��ɫ��Ϊ�ػ�ɫ 2H2O2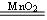 2H2O+
O2��
2H2O+
O2��
(3��H2S ��ˮ����ˮ�����Ը������ Ʒ��
����������1��Ũ��ˮ������ʯ�������ɰ����������ķ�Ӧ��CaO+NH3��H2O==Ca��OH��2+ NH3�����������Ȼ�����Ӧ��������������ɫ������
��2��˫��ˮ�ڶ������̵Ĵ������£���������������ʽΪ2H2O2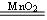 2H2O+
O2���������ܰ����������������������ӣ�����Һ��ɫ��dz��ɫ��Ϊ�ػ�ɫ��
2H2O+
O2���������ܰ����������������������ӣ�����Һ��ɫ��dz��ɫ��Ϊ�ػ�ɫ��
��3��SO2�ܰ������������ɵ�����Ҳ�ܻ�ԭ��ˮ����ˮ�����Ը��������Һ��SO2Ҳ����Ư���ԣ���ʹƷ����Һ��ɫ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ˮ��ѧ��һ��ѧ��һ�����Ի�ѧ�Ծ����������� ���ͣ������
����7��)ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺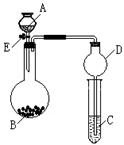
(1����AΪŨ��ˮ��BΪ�����ƣ�C��ʢ��AlCl3��Һ����������E���㹻��ʱ��۲쵽C�е�����Ϊ ��Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ ��
(2����AΪ30%��H2O2��Һ��BΪ�������̣�C��ʢ���ữ����FeCl2��Һ����������E��C�е�����Ϊ ��Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ ��
(3)��������װ�û�������֤SO2�Ļ�ѧ����, AΪ��Ũ���ᣬBΪ�������ƣ���C��ʢ��
��Һ����֤SO2��������; C��ʢ�� ��Һ����֤�仹ԭ��; ��C��ʢ�� ��Һ����֤��Ư���ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�������и�һ������ѧ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��ÿ��1�֣���7��)ij��ȤС����ʯ��ʯ��ϡ���ᷴӦ��ȡCO2������֤CO2�����ʡ���ȷ��װ�����������ú����ʵ�顣���Ƶõ�����ͨ�����ʯ��ˮ�У�����ʯ��ˮû�б���ǡ��Դ�չ��̽����
��1��[�������]����ʯ��ˮΪʲôˮû�б���ǣ�
[��������֤]�ټ�ͬѧ��Ϊ���ܳ���ʯ��ˮ�ѱ��ʡ���ȡ��������ʯ��ˮ���Թ��У������д�����������:__ ___��˵������ʯ��ˮû�б��ʡ�
����ͬѧ��Ϊ�Ƶõ�CO2�п�����HCl���塣�������˲����������_____________________�������Թ�ȡ������ɫʯ����Һ����ͨ���Ƶõ����壬��ɫʯ����Һ��죬���ó�������CO2�л������塣
�۱�ͬѧ��Ϊ��ͬѧ��ʵ������Ǵ���ģ�������_________________________����������������ʵ��֤����
[ʵ����֤]
| ʵ�鲽�� | ʵ������ | ʵ����� |
| | | CO2���HCl���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ��һ������ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ÿ��1�֣���7��)ij��ȤС����ʯ��ʯ��ϡ���ᷴӦ��ȡCO2������֤CO2�����ʡ���ȷ��װ�����������ú����ʵ�顣���Ƶõ�����ͨ�����ʯ��ˮ�У�����ʯ��ˮû�б���ǡ��Դ�չ��̽����
��1��[�������]����ʯ��ˮΪʲôˮû�б���ǣ�
[��������֤]�ټ�ͬѧ��Ϊ���ܳ���ʯ��ˮ�ѱ��ʡ���ȡ��������ʯ��ˮ���Թ��У������д�����������:__ ___��˵������ʯ��ˮû�б��ʡ�
�� ��ͬѧ��Ϊ�Ƶõ�CO2�п�����HCl���塣�������˲����������_____________________�������Թ�ȡ������ɫʯ����Һ����ͨ���Ƶõ����壬��ɫʯ����Һ��죬���ó�������CO2�л������塣
�۱�ͬѧ��Ϊ��ͬѧ��ʵ������Ǵ���ģ�������_________________________����������������ʵ��֤����
[ʵ����֤]
|
ʵ�鲽�� |
ʵ������ |
ʵ����� |
|
|
|
CO2���HCl���� |
��2����˼�����۶�ͬѧ��Ϊ��Ȼ��ϡ�����Ƶõ�CO2������Ϊ�β���H2SO4�������ᡣ���ʶ�ͬѧ���뷨����________��������_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ĸ�����ѧ����ĩ���ԣ����ۣ���ѧ���� ���ͣ�ʵ����
(���14��) ��ÿ��2�֣� ��ҵ�ϳ�����������ʢװ��Ũ���ᡣΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
��1������ȥ�������������������̼�ظ֣�������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ���� ��
��2������ȡ����6.0g����15.0ml��Ũ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ�������Y����ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+����Ҫȷ�����е�Fe2+��Ӧ���� ( ѡ����ţ�
a��KSCN��Һ����ˮ b�����ۺ�KSCN��Һ c��Ũ��ˮ d������KMnO4��Һ
��3������������2��ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ���H2��Q���塣Ϊ�����������̽��ʵ��״�ã�ͼ�мг�����ʡ�ԣ���

װ��B���Լ���������
��4����Ϊ����Y�л�����Q�������� ���û�ѧ����ʽ��ʾ����
��5��Ϊȷ��Q�Ĵ��ڣ�����װ��������M�� ��ѡ����ţ���
a�� A֮ǰ b��A-B�� c��B-C�� d��C-D��
��6���������Y�к���Q���壬Ԥ��ʵ�������� ��
��7���������Y�к���H2���壬Ԥ��ʵ�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com