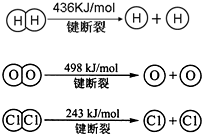
��2012?����ģ�⣩��Դ����������������ٵ��ش���⣬�ձ����������ĺ�й©�¹����������ǶԺ���Դ�Ŀֻţ����״���δ����Ҫ����ɫ��Դ֮һ����CH
4��H
2OΪԭ�ϣ�ͨ�����з�Ӧ���Ʊ��״���
��CH
4��g��+H
2O��g��=CO��g��+3H
2��g����H=+206.0kJ/mol
��CO��g��+2H
2��g��=CH
3OH��g����H=-129.0kJ/mol
��l��CH
4��g����H
2O��g����Ӧ����CH
3OH��g����H
2��g�����Ȼ�ѧ����ʽΪ
CH4��g��+H2O��g��=CH3OH��g��+H2��g����H=+77.0KJ/L
CH4��g��+H2O��g��=CH3OH��g��+H2��g����H=+77.0KJ/L
��
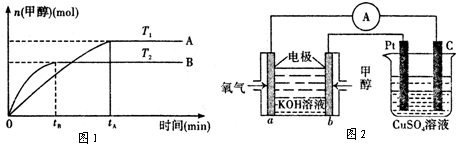
��2����1.0molCH
4��2.0molH
2O��g��ͨ���ݻ�Ϊ100L�ķ�Ӧ�ң���һ�������·�����ӦI�������һ����ѹǿ��CH
4��ת�������¶ȵĹ�ϵ��ͼ1��
�ټ���100��ʱ�ﵽƽ�������ʱ��Ϊ5min������H
2��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ
0.0024mol/L?min
0.0024mol/L?min
��
��100��ʱ��ӦI��ƽ�ⳣ��Ϊ
7.2��10-5��mol/L��2
7.2��10-5��mol/L��2
��
��3����ѹǿΪ0.1MPa���¶�Ϊ300�������£���1.0molCO��2.0molH
2�Ļ�������ڴ��������·�����Ӧ�����ɼ״���ƽ����������ݻ�ѹ����ԭ����
�������������䣬��ƽ����ϵ������Ӱ����
CD
CD
������ĸ��ţ���
A��c��H
2����С B������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
C��CH
3OH�����ʵ������� D������ƽ��ʱ
��С E��ƽ�ⳣ��K����
��4����ҵ�����ü״��Ʊ������ij��÷��������֣�
�ټ״��������������÷��е�һ����Ҫ��ӦΪCH
3OH��g��?CO��g��+2H
2��g�����˷�Ӧ���Է����е�ԭ���ǣ�
��Ӧ�������ӵķ�Ӧ
��Ӧ�������ӵķ�Ӧ
��
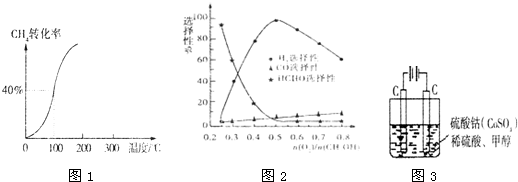
�ڼ״���������������һ���¶�����Ag/CeO
2-ZnOΪ����ʱԭ���������Է�Ӧ��ѡ���ԣ�ѡ����Խ��ʾ���ɵĸ�����Խ�ࣩӰ���ϵ��ͼ2��ʾ����n��O
2��/n��CH
3OH��=0.25ʱ��CH
3OH��O
2��������Ҫ��Ӧ����ʽΪ
��
��5���״���ˮ�ʻ����һ������Ⱦ����һ�ֵ绯ѧ��������������Ⱦ����ԭ���ǣ�ͨ���Co
2+������Co
3+��Ȼ����Co
3+����������ˮ�еļ״�������CO
2��������ʵ������ͼ3װ��ģ���������̣�
��д�������缫��Ӧʽ
Co2+-e-=Co3+
Co2+-e-=Co3+
��
��д����ȥ�״������ӷ���ʽ
6Co3++CH3OH+H2O=CO2��+6Co2++6H+��
6Co3++CH3OH+H2O=CO2��+6Co2++6H+��
��




 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g��
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g��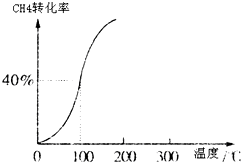 �ϳɰ���ũҵ���������������������Ҫ���壮
�ϳɰ���ũҵ���������������������Ҫ���壮