
�л���F�ĺϳ�·����ͼ��ʾ��
��֪������Ϣ��
(1)RCOOR�� RCH2OH
RCH2OH
(2)
�ش��������⣺
��1��A�Ľṹ��ʽΪ ��G�Ľṹ��ʽΪ ��
��2��B����C�Ļ�ѧ����ʽΪ ��
��3������E�к��������ŵ��Լ��� �������� ��
��4��E��F�ķ�Ӧ������ ��
��5��A��ͬ���칹���У���������������ͬ���칹���� �֣�������A����д������һ�ֵĽṹ��ʽ ��
�����ڷ����廯����
�ڱ��������ĸ�ȡ����
�۱����ϵ�һ��ȡ����ֻ��һ��
��1�� ��2�֣���
��2�֣��� ��2�֣�
��2�֣�
��2�� + 2CH3OH
+ 2CH3OH
 + 2H2O��2�֣�
+ 2H2O��2�֣�
��3��������Һ������������ͭ����Һ��1�֣��Թܱڸ��Ź������������ש��ɫ������2�֣�
��4���ӳɷ�Ӧ��1�֣�
��5��5�֣�3�֣� ��2�֣����������𰸾����֣�
�������������A�ܱ����Ը��������Һ��������2���Ȼ�����������֪��Ϣ��֪A��Ӧ�ú���2����������F�Ľṹ��ʽ��֪A�е�2����Ӧ������λ�ģ����A�Ľṹ��ʽΪ ����B�Ľṹ��ʽΪ
����B�Ľṹ��ʽΪ ��B��C����������Ӧ����C����C�Ľṹ��ʽΪ
��B��C����������Ӧ����C����C�Ľṹ��ʽΪ ��B�����к���2���Ȼ������Ҷ����������۷�Ӧ���ɸ߷��ӻ�����G����G�Ľṹ��ʽΪ
��B�����к���2���Ȼ������Ҷ����������۷�Ӧ���ɸ߷��ӻ�����G����G�Ľṹ��ʽΪ ��������֪��Ϣ��֪C����D����D�Ľṹ��ʽΪ
��������֪��Ϣ��֪C����D����D�Ľṹ��ʽΪ ��D��������������E����E�Ľṹ��ʽΪ
��D��������������E����E�Ľṹ��ʽΪ ��E��˫��ˮ������������F��
��E��˫��ˮ������������F��
��1���������Ϸ�����֪A�Ľṹ��ʽΪ ��G�Ľṹ��ʽΪ
��G�Ľṹ��ʽΪ ��
��
��2��B����C��������Ӧ����Ӧ�Ļ�ѧ����ʽΪ + 2CH3OH
+ 2CH3OH
 + 2H2O��
+ 2H2O��
��3��E�к���ȩ������������������Һ�����Ƶ�������ͭ����Һ���飬��˼���E�к��������ŵ��Լ���������Һ������������ͭ����Һ��ʵ���������Թܱڸ��Ź������������ש��ɫ������
��4��E�к���ȩ����F��ȩ����Ϊ�ǻ�����˵��E��F�ķ�Ӧ������ȩ���ļӳɷ�Ӧ��
��5�����ڷ����廯����˵�����б��������������ĸ�ȡ�����ұ����ϵ�һ��ȡ����ֻ��һ�֣���˵��ȡ����Ӧ���ǶԳƵģ���˷���������A��ͬ���칹��Ľṹ��ʽΪ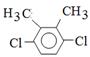 ��
�� ��
�� ��
�� ��
�� ��������5�֡�
��������5�֡�
���㣺�����л����ƶϡ��л���Ӧ���͡�ͬ���칹���ж��Լ��йط���ʽ����д


 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��16�֣�����Һ�����л���III��Ӧ��ż����Ӧ�ϳɣ�
��Ӧ��
��Ӧ��
�л���I�������ºϳɣ��л���IV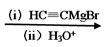 �л���I
���I
�Իش��������⣺
���л���I�ķ���ʽ�� ��
���Ա�Ϊԭ�������л���II�Ļ�ѧ����ʽ��ע����Ӧ�������� ��
���л���IV�Ľṹ��ʽ�� ��
���л���IV�ϳ��л���I�ĵ�һ����Ӧ�������ķ�Ӧ������ ��
�����л���III�Ʊ� 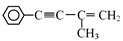 �ķ�Ӧ������ ��
�ķ�Ӧ������ ��
��д���������������Ļ�����III��ͬ���칹��Ľṹ��ʽ�� ����дһ����
�ٺ���һ���������ұ�����һ��ȡ������2��ͬ���칹�塣 ���ܷ���������Ӧ
�� ��CH3I�ܷ������Ƣٵķ�Ӧ���÷�Ӧ����ʽ��
��CH3I�ܷ������Ƣٵķ�Ӧ���÷�Ӧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����A����ʽΪC3H4O2�������ԡ�FΪ���߸�ԭ����ɵĻ�״�ṹ������ʽΪC6H8O4 ����������¿�ͼ�ش�����
��1��A�Ľṹ��ʽΪ
��2����Ӧ�ٵķ�Ӧ����Ϊ
��3��������B�к��������ŵ�������
��4��D��E����F�Ļ�ѧ����ʽ
D��E����1:1��ӦҲ�����ɸ߾����д�����ɸø߾���Ļ�ѧ��Ӧ����ʽ��
��5��G����H�Ļ�ѧ����ʽ
��6��д��C��ͬ���칹���������������ʵĽṹ��ʽ �� �� ������д3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���A��̼���⡢������Ԫ����ɡ�Ϊ�о�A�������ṹ������������ʵ�飺
��ȡA 9.0g������ʹ�������������ܶ�����ͬ������H2��45��������9.0gA��������O2�г��ȼ�գ���ʹ���������ͨ����ʯ�ҡ���ˮ����ͭ��ĩ������ʯ��ˮ�����ּ�ʯ������14.2g������ͭ��ĩû�б�����ʯ��ˮ����10.0g��ɫ�������ɣ������صļ�ʯ���м��������������4.48L��ɫ��ζ���壨��״��������ȡA 9.0g������������NaHCO3��ĩ��Ӧ������2.24LCO2����״�������������������Ʒ�Ӧ������2.24LH2����״�������л���A����������������Ӧ������Ԫ������B
��ͨ��������գ���1���л���A����Է�������Ϊ_____________ ��
��2���л���A�ķ���ʽ_____________��
��3��A�Ľṹ��ʽ _____________ ��B�Ľṹ��ʽ _____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Լ�����������( )�����ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����
)�����ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����
(1)D�к��й����ŵ�������________��A��E�ķ�Ӧ����Ϊ______��
(2)G�Ľṹ��ʽΪ_______��
(3)д��1�����������ұ�����ֻ��һ��ȡ������C8H8O2��ͬ���칹�� ��
(4)�����( )�ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________(�����)��
)�ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________(�����)��
| A��NaOH��Һ | B��NaHCO3��Һ | C��KMnO4/H+ | D��FeCl3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij������A��һ����Ҫ���л�����ԭ�ϣ�����Ϊ��ʼԭ�Ͼ�������ת����ϵ�����ֲ���ϳ�·�ߡ���Ӧ������ȥ�����Ժϳ��ڰ��������ᡢ����������ʡ�����D����������Һ����������Ӧ��H��һ�ֹ��ܸ߷��ӡ�

��1��д��������Ľṹ��ʽ�� ��д��G�����ŵ����� ��
��2����Ӧ�ڵķ�Ӧ����Ϊ�� ��������Ӧ�ܵ�����Ϊ���� ��
��Ӧ�������ܲ��ܶԻ����������� ��
��3��д��D��������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��4��д����Ӧ�ݵĻ�ѧ����ʽ�� ��
��5��д��������������G��ͬ���칹��Ľṹ��ʽ �� ��(��д����)
�ٺ��б������ұ����ϵ�һ��ȡ������ֻ�ж��֣�
�ڱ�����ֻ������ȡ��������Nԭ��ֱ���뱽��������
�۽ṹ�в������ǻ��ͷ��ǻ���
��6������ƺ����ķ�������ϩΪ��Ҫ�л�ԭ�Ϻϳɡ�
��ʾ���ٺϳɹ��������Լ���ѡ���� �ϳ�·������ͼʾ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ȡ9��20gֻ���ǻ����������������ŵı��Ͷ�Ԫ�������������������У�����ȼ������ȫȼ�գ�ȼ�պ�����徭��Ũ����ʱ��Ũ��������7��20g��ʣ�����徭CaO���պ��������6��72L����״���²ⶨ����
��1��9��20g����C��H��O�����ʵ����ֱ�Ϊ��C mol��H mol��O mol���ô���C��H��O��ԭ�Ӹ���֮��Ϊ ��
��2�������ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ ����ԭ���� ��
��3��������һ��̼ԭ�ӵı���һԪ���ܴ�������ȩ�����������ĸô��Ľṹ��ʽ�У� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ϩ����̼̼˫������Ҫ�Ĺ����ţ��ڲ�ͬ�������ܷ������ֱ仯��
(1)ϩ���ĸ��ֽⷴӦ��������ϩ������˫�����˵Ļ��ţ�����������ϩ���ķ�Ӧ����д���ڴ��������£�������ϩ���Ӽ䷢�����ֽⷴӦ�Ļ�ѧ����ʽ____________________________��
(2)ϩ����������������Һ��Ӧ���������������µķ�Ӧ��ϵ��
��֪ijϩ���Ļ�ѧʽΪC5H10������������������Һ��Ӧ��õ��IJ�����Ϊ����ͱ��ᣬ���ϩ���Ľṹ��ʽ��________________����Ϊ������̼�Ͷ�ͪ(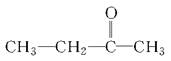 )�����ϩ���Ľṹ��ʽ��________��
)�����ϩ���Ľṹ��ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���۷��ɸĽ��л��߷��ӻ���������ʣ��߷��Ӿۺ���P�ĺϳ�·�����£�

��1��A�ĽṹʽΪ________________ ��C������Ϊ
��2��D�к��еĹ����ŵ�����
��3����F���١��ۺϳ�I��F����ʹ��ˮ��ɫ����Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ڵķ�Ӧ������ ��Ӧ�۵ķ�Ӧ�Լ��� ��
��4������˵����ȷ���� ��
a��C����ˮ����������
b��A��1,3-����ϩ��Ϊͬϵ��
c����I����Mʱ��1mol I�������3molNaOH
d��N������˳���칹��
��5��д����D����E�Ļ�ѧ����ʽ ��
��6��E��N�����ʵ����������۷�Ӧ����P����P�Ľṹ��ʽΪ ��
��7��E�ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ ��
a��������������ȡ����
b����ʹFeCl3Һ����ɫ
c��1mol���л�����Ũ��ˮ��Ӧʱ������4molBr2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com