
���ǵ����ϼ�Ϊ�ḻ��Ԫ�ء�
��1��N2�Ǵ�������Ҫ�ɷ�֮һ�����ڷ����м��ܴܺ����������ȶ�����֪N��N�ļ���Ϊ946 kJ��mol��1��N��N�����ļ���Ϊ193kJ��mol��1��
���㣺N2�����С��С����ļ���ԼΪ ��
���ۣ�N2�����С��ҡ��͡��С������ȶ��� ��
��2�������������Ǵ�����Ⱦ��֮һ��Ϊ��������Ⱦ��������Ա�����ͬʱ������������͵���������ķ������乤���������£�
��������ҡ��ֽ��ҷ����ķ�Ӧ���£�
����ң�NO + NO2 = N2O3 N2O3 + 2H2SO4 = 2NOHSO4+ H2O
�ֽ��ң�4NOHSO4 + O2 + 2H2O = 4H2SO4+ 4NO2
�ش��������⣺
�ٺ͢ڷֱ�Ϊ��д��ѧʽ�� �� ��
�������ҷ����ķ�Ӧ�� ��
��3��������������һ����Ҫ�Ļ�ѧ���ʣ������������;��ij�������ӣ�M+����N3���γɵľ���ṹ����ͼ��ʾ������M+�����е������ó���K��L��M�������Ӳ㣬��M+�ķ����� ����ͬһ��N3��������M+�� ����
��4��NH3������Ҫ�Ĺ�ҵ��Ʒ��������Ҫ�Ĺ�ҵԭ�ϡ���NH3Ϊԭ����������淋Ĺ�
�����£�
���з�Ӧ��Ϊ��4NO+3O2+2H2O=4HNO3 ԭ����Ϊ�����Ϳ����Ļ�������������������������Ϊ0.2��
��д����Ӧ�ٵĻ�ѧ����ʽ ���������Ǹ���Ӧ�Ҹ�����Ӧ����ȫ������������ԭ�����еİ������������ڢ۲����������յİ������Ϳ���������ǡ��ȫ��ת��Ϊ���ᣬ��ԭ�������Ʊ�����İ����������������Ϊ ��
����ʵ�������У���Ӧ���а���ת���ʣ��������ʣ�Ϊ70%����Ӧ����NO��ת����Ϊ90%����Ӧ���а����������ȫת��������������İ���ռ���ð����������������Ϊ���٣���д��������̣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
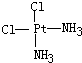 ��
��
 ��
��
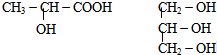
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧ�� | N-N | N=N | N��N | N-H | H-H |
| ����/kJ?mol-1 | 159 | 418 | 946 | 391 | 436 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com