
����(�Է�ĩ״����)�Ͱ�������������ͬ�������塣
��֪��
��Sn(s����)��2HCl(aq)===SnCl2(aq)��H2(g)����H1
��Sn(s����)��2HCl(aq)===SnCl2(aq)��H2(g)����H2
��Sn(s����)  Sn(s����)
Sn(s����)
��H3����2.1 kJ��mol��1
����˵����ȷ����(����)
A����H1>��H2
B�����ڳ������Ի���״̬����
C������ת��Ϊ�����ķ�Ӧ�Ƿ��ȷ�Ӧ
D�����������ڴ��ڵ���13.2��Ļ����У������лٻ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���������Ĺ�ϵ�dz����У����ִ����ĸ��������л��ﶼ������Ҫ�����á������������ʣ����顢�����顢��ϩ����Ȼ�����������Ա����ӡ���ȩ��ţ�͡���ѡ����ȷ����������Ӧ�Ŀո��
(1)���ڱ���������____________________________________________��
(2)���ڷ����廯�������______________________________________��
(3)���ڻ������� _________________________________________��
(4)�����к���̼̼˫����������������__________________________��
(5)��ϵͳ���������Ա�����������______________________________��
(6)��ȩ����������Ӧ�Ļ�ѧ����ʽ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һԪ����ͨʽΪ ����nֵ�� ʱ���ô���ʼ���ִ����ͬ���칹�壻��nֵ�� ʱ���ô���ʼ���ַǴ����ͬ���칹�壻��nֵ�� ʱ���ô����ܱ����������ﲻ��ȩ��ͬ���칹�壻��nֵ�� ʱ���ô��ĸ���ͬ���칹��ֱ���Ũ���Ṳ�Ⱥ�ֻ�õ�һ��ϩ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2008�걱���а»Ἢ��������ţΪ������Ƶġ���ţ���֡�(Funiulele)����һ���л���ļ���ʽҲ����ţ���ʳ�Ϊţʽ��ϩȲ��(cowenynenynol)�������й�˵������ȷ����(����)
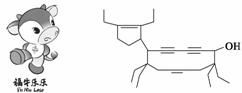
A��ţʽ��ϩȲ�������ں������ֹ�����
B��ţʽ��ϩȲ���ܹ������Ӿ۷�Ӧ�õ��߷��ӻ�����
C��ţʽ��ϩȲ����һ���������������ᷢ��������Ӧ
D��ţʽ��ϩȲ����ʹ���Ը��������Һ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
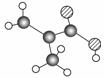
(8��)ij����ֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ�������й���12��ԭ��(ͼ��������֮������ߴ���������˫���Ȼ�ѧ��)��
(1)�����ʵĽṹ��ʽΪ_____________________________________��
(2)�����������������ŵ�����Ϊ_______________________________________��
(3)���������У���ò�Ʒ��Ϊͬϵ�����(�����)____________����Ϊͬ���칹�����________________________________________________________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪H2(g)��C2H4(g)��C2H5OH(l)��ȼ���ȷֱ��ǣ�285.8 kJ��mol��1����1 411.0 kJ��mol��1�ͣ�1 366.8 kJ��mol��1������C2H4(g)��H2O(l)��Ӧ����C2H5OH(l)�Ħ�HΪ(����)
A����44.2 kJ��mol��1 B����44.2 kJ��mol��1
C����330 kJ��mol��1 D����330 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һ����̼�����顢����ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ
H2(g)�� O2(g)===H2O(l)�� ��H����285.8 kJ��mol��1
O2(g)===H2O(l)�� ��H����285.8 kJ��mol��1
CO(g)�� O2(g)===CO2(g)�� ��H����283.0 kJ��mol��1
O2(g)===CO2(g)�� ��H����283.0 kJ��mol��1
C8H18(l)�� O2(g)===8CO2(g)��9H2O(l) ��H����5 518 kJ��mol��1
O2(g)===8CO2(g)��9H2O(l) ��H����5 518 kJ��mol��1
CH4(g)��2O2(g)===CO2(g)��2H2O(l) ��H����890.3 kJ��mol��1
��ͬ������������һ����̼�����顢������ȫȼ��ʱ���ų��������ٵ���(����)
A��H2 B��CO C��C8H18 D��CH4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����к��м��Լ������ڸþ����˵��������ǣ� ��
A���������кܸߵ��۷е� B���������ǵ���
C���������л��� D�����������Ӿ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С�飬�����µ�һ���п�������Լ�ƿ�У�����������ʳ��ˮ�����ʪ���ټ����η�̪��Һ������ͼװ�ý���ʵ�飬�����Ӻ�۲죬���������ܳ��ֵ���(����)

A����������������
B�����е��������γ�һ��ˮ��
C������Ƭ���ڴ���Һ���
D��п����ʴ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com