
(12·Ö)ÓĆĻĀĆęĮ½ÖÖ·½·ØæÉŅŌÖʵư×É«µÄFe(OH)2³Įµķ”£
·½·ØŅ»£ŗÓĆ²»ŗ¬Fe3+µÄFeSO4ČÜŅŗÓėÓĆ²»ŗ¬O2µÄÕōĮóĖ®ÅäÖʵÄNaOHČÜŅŗ·“Ó¦Öʱø”£
£Ø1£©ÓĆĮņĖįŃĒĢś¾§ĢåÅäÖĘÉĻŹöFeSO4ČÜŅŗŹ±»¹Šč¼ÓČėĻ”H2SO4ŗĶ______”£
£Ø2£©³żČ„ÕōĮóĖ®ÖŠČܽāµÄO2³£²ÉÓĆ____________µÄ·½·Ø”£
£Ø3£©Éś³É°×É«Fe(OH)2³ĮµķµÄ²Ł×÷ŹĒÓĆ³¤µĪ¹ÜĪüČ”²»ŗ¬O2µÄNaOHČÜŅŗ£¬²åČėFeSO4ČÜŅŗŅŗĆęĻĀŌŁ¼·³öNaOHČÜŅŗ”£ÕāŃł²Ł×÷µÄĄķÓÉŹĒ_______________________________”£
·½·Ø¶ž£ŗŌŚČēĶ¼ĖłŹ¾×°ÖĆÖŠ£¬ÓĆNaOHČÜŅŗ”¢ĢśŠ¼”¢Ļ”H2SO4µČŹŌ¼ĮÖʱø”£
£Ø1£©ŌŚŹŌ¹Ü¹¤Ąļ¼ÓČėµÄŹŌ¼ĮŹĒ_______________”£
£Ø2£©ŌŚŹŌ¹Ü¢ņĄļ¼ÓČėµÄŹŌ¼ĮŹĒ_______________”£
£Ø3£©ĪŖĮĖÖʵư×É«Fe(OH)2³Įµķ£¬ŌŚŹŌ¹Ü¢ńŗĶ¢ņÖŠ¼ÓČėŹŌ¼Į£¬“ņæŖÖ¹Ė®¼Š£¬Čū½ōČū×ÓŗóµÄŹµŃé²½ÖčŹĒ_________________________________”£
£Ø4£©Õāѳɜ³ÉµÄFe(OH)2³ĮµķÄܽĻ³¤Ź±¼ä±£³Ö°×É«£¬ĘäĄķÓÉŹĒ________________________________________________________________”£
 ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
2009Äź12ŌĀ18ČÕ·ļ»ĖĶų±ØµĄ£¬¾¹ż±±¾©¶„¼¶ÖŠĪ÷Ņ½×ؼŅ³¤“ļ7øöŌĀµÄæĘŃŠ¹„¹Ų£¬ÖÕÓŚÖĘ³öŅ»ÖÖÓŠŠ§ÖĪĮĘ¼×ŠĶH1N1Į÷øŠµÄŠĀÖŠŅ©”°½š»ØĒåøŠ·½”±”£ĘäÖ÷ŅŖ³É·ÖÖ®Ņ»ŹĒ½šŅų»Ø£¬¶ų½šŅų»ØµÄÓŠŠ§³É·ÖÖ®Ņ»ŹĒĀĢŌĖį”£ĻĀĆęŹĒÓŠ¹ŲĀĢŌĖįµÄ·Ö×Ó½į¹¹¼°Ęä×Ŗ»Æ¹ŲĻµ£¬ĘäÖŠEĪŖ·¼ĻćĢž£¬Ęä±½»·ÉĻµÄŅ»ĀČ“śĪļÖ»ÓŠŅ»Ö֣زæ·Ö²śĪļŹ”ĀŌ£©


Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĀĢŌĖįµÄ·Ö×ÓŹ½ŹĒ £»AÖŠŗ¬Ńõ¹ŁÄÜĶŵÄĆū³ĘĪŖ £»HµÄ½į¹¹¼ņŹ½ĪŖ ”£
£Ø2£©¢ŁµÄ·“Ó¦ĄąŠĶŹĒ £»1molĀĢŌĖį×ī¶ąĻūŗÄ molNaOH”£
£Ø3£©·¼ĻćĢžEµÄĶ¬·ÖŅģ¹¹Ģå£ØÓėEŗ¬ÓŠĻąĶ¬µÄČ”“ś»ł£©µÄ·ŠµćÓɓ󵽊”µÄĖ³ŠņŹĒ£Ø°üĄØEŌŚÄŚ£© £ØÓĆ½į¹¹¼ņŹ½±ķŹ¾£©”£
£Ø4£©Š“³ö·“Ó¦¢Ū”¢¢ÜµÄ»Æѧ·½³ĢŹ½£ŗ
¢Ū £»
¢Ü ”£
£Ø5£©BŹĒ槷ČĖį£¬ĖüÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬Š“³öĀś×ćĻĀĮŠĢõ¼žµÄĖłÓŠĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ£ØĢõ¼ž£ŗa.±½»·ÉĻŗ¬ÓŠĮ½øöČ”“ś»ł£¬ĒŅ±½»·ÉĻµÄŅ»ĀČ“śĪļÖ»ÓŠĮ½ÖÖ£»b.ÄÜ·¢ÉśĖ®½ā·“Ó¦£¬ĒŅ²śĪļÖ®Ņ»ÄÜ·¢ÉśŅų¾µ·“Ó¦£»c.ÄÜÓėĢ¼ĖįĒāÄĘČÜŅŗ·“Ó¦£© ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(12·Ö)ÓĆĻĀĆęĮ½ÖÖ·½·ØæÉŅŌÖʵư×É«µÄFe(OH)2³Įµķ”£
·½·ØŅ»£ŗÓĆ²»ŗ¬Fe3+µÄFeSO4ČÜŅŗÓėÓĆ²»ŗ¬O2µÄÕōĮóĖ®ÅäÖʵÄNaOHČÜŅŗ·“Ó¦Öʱø”£
£Ø1£©ÓĆĮņĖįŃĒĢś¾§ĢåÅäÖĘÉĻŹöFeSO4ČÜŅŗŹ±»¹Šč¼ÓČėĻ”H2SO4ŗĶ______”£
£Ø2£©³żČ„ÕōĮóĖ®ÖŠČܽāµÄO2³£²ÉÓĆ____________µÄ·½·Ø”£
£Ø3£©Éś³É°×É«Fe(OH)2³ĮµķµÄ²Ł×÷ŹĒÓĆ³¤µĪ¹ÜĪüČ”²»ŗ¬O2µÄNaOHČÜŅŗ£¬²åČėFeSO4ČÜŅŗŅŗĆęĻĀŌŁ¼·³öNaOHČÜŅŗ”£ÕāŃł²Ł×÷µÄĄķÓÉŹĒ_______________________________”£
·½·Ø¶ž£ŗŌŚČēĶ¼ĖłŹ¾×°ÖĆÖŠ£¬ÓĆNaOHČÜŅŗ”¢ĢśŠ¼”¢Ļ”H2SO4µČŹŌ¼ĮÖʱø”£
£Ø1£©ŌŚŹŌ¹Ü¹¤Ąļ¼ÓČėµÄŹŌ¼ĮŹĒ_______________”£
£Ø2£©ŌŚŹŌ¹Ü¢ņĄļ¼ÓČėµÄŹŌ¼ĮŹĒ_______________”£
£Ø3£©ĪŖĮĖÖʵư×É«Fe(OH)2³Įµķ£¬ŌŚŹŌ¹Ü¢ńŗĶ¢ņÖŠ¼ÓČėŹŌ¼Į£¬“ņæŖÖ¹Ė®¼Š£¬Čū½ōČū×ÓŗóµÄŹµŃé²½ÖčŹĒ_________________________________”£
£Ø4£©Õāѳɜ³ÉµÄFe(OH)2³ĮµķÄܽĻ³¤Ź±¼ä±£³Ö°×É«£¬ĘäĄķÓÉŹĒ________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģĮÉÄžŹ”ĮÉÄĻŠ×÷ĢåøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
(12·Ö)ŌŚŅ»¶ØĪĀ¶ČĻĀ½« 2 mol AŗĶ2 mol BĮ½ÖÖĘųĢå»ģŗĻÓŚ2 LĆܱÕČŻĘ÷ÖŠ£¬·¢ÉśČēĻĀ·“Ó¦£ŗ3A(g)£«B(g)
2 mol AŗĶ2 mol BĮ½ÖÖĘųĢå»ģŗĻÓŚ2 LĆܱÕČŻĘ÷ÖŠ£¬·¢ÉśČēĻĀ·“Ó¦£ŗ3A(g)£«B(g) 2C(g)£«2D(g),2·ÖÖÓÄ©·“Ó¦“ļµ½Ę½ŗāדĢ¬£¬Éś
2C(g)£«2D(g),2·ÖÖÓÄ©·“Ó¦“ļµ½Ę½ŗāדĢ¬£¬Éś ³ÉĮĖ0.8 mol D£¬ĒėĢīŠ“ĻĀĆęæÕ°×”£
³ÉĮĖ0.8 mol D£¬ĒėĢīŠ“ĻĀĆęæÕ°×”£
(1) BµÄĘ½ŗāÅضČĪŖ________”£
(2) AµÄ×Ŗ»ÆĀŹĪŖ________”£
(3) ÓĆD±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ________”£
(4) Čē¹ūĖõŠ”ČŻĘ÷ČŻ»ż(ĪĀ¶Č²»±ä)£¬“ļµ½ŠĀĘ½ŗāŹ±»ģŗĻĘųĢåµÄĆܶČ________”¢Ę½¾łĻą¶Ō·Ö×ÓÖŹĮæ________”£(Į½æÕ¾łŃ”Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
(5)Čē¹ūÉĻŹö·“Ó¦ŌŚĻąĶ¬Ģõ¼žĻĀ“ÓÄę·“Ó¦æŖŹ¼½ųŠŠ£¬æŖŹ¼¼ÓCŗĶDø÷4/3 mol£¬ŅŖŹ¹Ę½ŗāŹ±ø÷ĪļÖŹµÄÖŹĮæ·ÖŹżÓėŌĘ½ŗāŹ±ĻąµČ£¬Ōņ»¹Ó¦¼ÓČė________mol BĪļÖŹ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźŠĀæĪ±ź»Æѧ±ŲŠŽŅ»µŚČżÕĀ½šŹō¼°Ęä»ÆŗĻĪļµ„ŌŖ²āŹŌ£ØČĖ½Ģ°ę£© ĢāŠĶ£ŗĢīæÕĢā
(12·Ö)ÓĆĻĀĆęĮ½ÖÖ·½·ØæÉŅŌÖʵư×É«µÄFe(OH)2³Įµķ”£
·½·ØŅ»£ŗÓĆ²»ŗ¬Fe3+µÄFeSO4ČÜŅŗÓėÓĆ²»ŗ¬O2µÄÕōĮóĖ®ÅäÖʵÄNaOHČÜŅŗ·“Ó¦Öʱø”£
£Ø1£©ÓĆĮņĖįŃĒĢś¾§ĢåÅäÖĘÉĻŹöFeSO4ČÜŅŗŹ±»¹Šč¼ÓČėĻ”H2SO4ŗĶ______”£
£Ø2£©³żČ„ÕōĮóĖ®ÖŠČܽāµÄO2³£²ÉÓĆ____________µÄ·½·Ø”£
£Ø3£©Éś³É°×É«Fe(OH)2³ĮµķµÄ²Ł×÷ŹĒÓĆ³¤µĪ¹ÜĪüČ”²»ŗ¬O2µÄNaOHČÜŅŗ£¬²åČėFeSO4ČÜŅŗŅŗĆęĻĀŌŁ¼·³öNaOHČÜŅŗ”£ÕāŃł²Ł×÷µÄĄķÓÉŹĒ_______________________________”£
·½·Ø¶ž£ŗŌŚČēĶ¼ĖłŹ¾×°ÖĆÖŠ£¬ÓĆNaOHČÜŅŗ”¢ĢśŠ¼”¢Ļ”H2SO4µČŹŌ¼ĮÖʱø”£
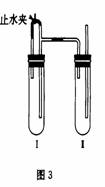
£Ø1£©ŌŚŹŌ¹Ü¹¤Ąļ¼ÓČėµÄŹŌ¼ĮŹĒ_______________”£
£Ø2£©ŌŚŹŌ¹Ü¢ņĄļ¼ÓČėµÄŹŌ¼ĮŹĒ_______________”£
£Ø3£©ĪŖĮĖÖʵư×É«Fe(OH)2³Įµķ£¬ŌŚŹŌ¹Ü¢ńŗĶ¢ņÖŠ¼ÓČėŹŌ¼Į£¬“ņæŖÖ¹Ė®¼Š£¬Čū½ōČū×ÓŗóµÄŹµŃé²½ÖčŹĒ_________________________________”£
£Ø4£©Õāѳɜ³ÉµÄFe(OH)2³ĮµķÄܽĻ³¤Ź±¼ä±£³Ö°×É«£¬ĘäĄķÓÉŹĒ________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com