
(��12��)ijѧ����������װ��̽�������백��֮��ķ�Ӧ������A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ��������������백����Ӧ��װ�á�ʵ���ҿ�����װ��F�Ʊ���������Ӧ�Ļ�ѧ����ʽΪ��MnO2��4HCl(Ũ) MnCl2��Cl2����2H2O
��ش��������⣺
(1)��װ��F�з�����Ӧ�����ӷ���ʽΪ_______________________________________��
(2)��Ϊ�˿����Ʊ�������װ��A����ƿ�п�װ�Լ�_____________________��
(3)��Bװ�õ�������_____________��(�����) A.ϴ��ƿ B.����� C.��Һ©��
Eװ�õ�������__________________________________________________________��
(4)ͨ��Cװ�õ�����������߽ϳ����ұ߽϶̣�Ŀ����________________________��
(5)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ����д����Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
(6)��װ��C��G���ݳ���β���п��ܺ��л���ɫ���ж����壬��δ�����____________________________________________________________________________��
(1)MnO2��4H����2Cl��Mn2����Cl2����2H2O��2�֣�
(2).CaO��.NaOH��1�֣�
(3)B����1�֣� ��ȥ�����е��Ȼ��⣨2�֣�
(4)ʹ�ܶȴ���������ܶ�С�İ����Ͽ�ؾ��Ȼ�ϣ�2�֣�
(5)3Cl2��8NH3===N2��6NH4Cl��2�֣�
(6)��G���ӵ���ֱ��ͨ��ʢ���ռ���ձ��У�2�֣�
����:��1��F������ȡ�����ģ�����ʽΪMnO2��4H����2Cl��Mn2����Cl2����2H2O��
��2����ˮ���лӷ���Ũ��Խ��Խ�ӷ�����������ܽ�����¶ȵ����߶����ͣ����Կ��Խ���ˮ������ʯ�һ��������ƹ����С�
��3������Bװ���ص���жϣ��������Ǹ���ܡ���ȡ�������к����Ȼ������壬������Ҫ���ñ���ʯ��ˮ����ȥ�����Ȼ��⡣
��4���������ܶ�С�ڿ����ģ��������ܶȴ��ڿ����ģ�������������Ŀ����Ϊ��ʹ�����ֻ�ϡ�
��5����������ǿ�����ԣ�����������������ʵ��������ж��������ǵ������Ȼ�泥�����ʽΪ3Cl2��8NH3===N2��6NH4Cl��
��6�������ж�����Ҫβ���������������ü�Һ�����ն����������


 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�����к����ѧ������һ���¿���ѧ�Ծ����������� ���ͣ������
(���12��)ij��ɫ����Һ���ܺ����������ӣ�K����Al3����Fe3����Ba2����NO3-��SO42-��HCO3-��Cl����ȡ����Һ��������ʵ�飺
������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬��������������������Ϊ����ɫ��
��ȡ��Һ���������백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ��
��ȡ��Һ�����������Ȼ�����Һ������ɫ������
��ȡʵ��ܺ�ij�����Һ��������������Һ������ɫ�������ټ��������ϡ���ᣬ��������ʧ��
��ش��������⣺
(1)��ʵ����У���ͼ��ʾ�IJ�������ȷ����________(�����)
(2)��������ʵ���ж�ԭ��Һ�п϶����ڵ�������________���϶������ڵ�������________��
(3)д����ڢ�����ʵ���йص����ӷ���ʽ��
��________________________________����_____________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡɽһ�и���10���¿���ѧ�Ծ� ���ͣ�ʵ����
(12��)ijѧ����������װ��̽�������백��֮��ķ�Ӧ������A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ��������������백����Ӧ��װ�á�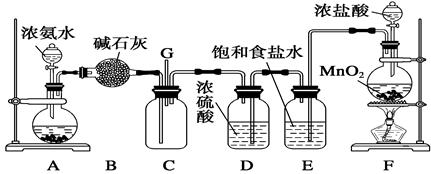
��ش��������⣺
(1)װ��F�з�����Ӧ�����ӷ���ʽΪ____________________________________________��
(2) Bװ�õ�������___________________��Eװ�õ�����___________________________��
(3)ͨ��Cװ�õ�����������߽ϳ����ұ߽϶̣�Ŀ����____________________________��
(4)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ����д����Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ���ڸ���ѧ��һ��ѧ�����л�ѧ�Ծ����������� ���ͣ�ʵ����
(��12��)ijѧ����������װ��̽�������백��֮��ķ�Ӧ������A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ��������������백����Ӧ��װ�á�ʵ���ҿ�����װ��F�Ʊ���������Ӧ�Ļ�ѧ����ʽΪ��MnO2��4HCl(Ũ)MnCl2��Cl2����2H2O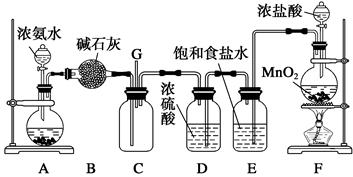
��ش��������⣺
(1)��װ��F�з�����Ӧ�����ӷ���ʽΪ_______________________________________��
(2)��Ϊ�˿����Ʊ�������װ��A����ƿ�п�װ�Լ�_____________________��
(3)��Bװ�õ�������_____________��(�����) A.ϴ��ƿ B.����� C.��Һ©��
Eװ�õ�������__________________________________________________________��
(4)ͨ��Cװ�õ�����������߽ϳ����ұ߽϶̣�Ŀ����________________________��
(5)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ����д����Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
(6)��װ��C��G���ݳ���β���п��ܺ��л���ɫ���ж����壬��δ�����____________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ʵ����
(��12��)ijѧ����������װ��̽�������백��֮��ķ�Ӧ������A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ��������������백����Ӧ��װ�á�ʵ���ҿ�����װ��F�Ʊ���������Ӧ�Ļ�ѧ����ʽΪ��MnO2��4HCl(Ũ) MnCl2��Cl2����2H2O
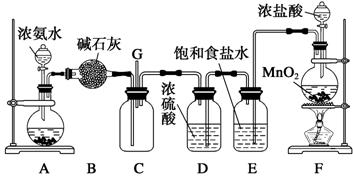
��ش��������⣺
(1)��װ��F�з�����Ӧ�����ӷ���ʽΪ_______________________________________��
(2)��Ϊ�˿����Ʊ�������װ��A����ƿ�п�װ�Լ�_____________________��
(3)��Bװ�õ�������_____________��(�����) A.ϴ��ƿ B.����� C.��Һ©��
Eװ�õ�������__________________________________________________________��
(4)ͨ��Cװ�õ�����������߽ϳ����ұ߽϶̣�Ŀ����________________________��
(5)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ����д����Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
(6)��װ��C��G���ݳ���β���п��ܺ��л���ɫ���ж����壬��δ�����____________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com