
����ѧ����ѡ��5���л���ѧ������ ��15�֣�
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���ú�D����Է���������ȣ���EΪ��֧���Ļ����
������ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ����C�����еĹ����������� ______________��������B���ܷ����ķ�Ӧ�ǣ�����ĸ��ţ���______________
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ
d��������Ӧ e��ˮ�ⷴӦ f���û���Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��__________________��
��3����Ӧ��ʵ���м��ȵ�Ŀ���ǣ� .��
��4��A�Ľṹ��ʽ�� __________________��
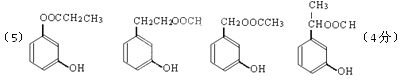
��5��д��ͬʱ������������������B��ͬ���칹������ͬ���칹��Ľṹ��ʽ��
���м��ȡ�������ṹ
�����ڷ������γɵ���
���� FeCl3��Һ������ɫ��Ӧ
��1���Ȼ� ��2�֣�
e ��2�֣�
��2��CH3COOH+CH3CH2CH2OH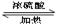 CH3COOCH2CH2CH3+H2O��2�֣�
CH3COOCH2CH2CH3+H2O��2�֣�
��3���ټӿ췴Ӧ����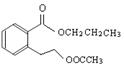
 �ڽ������������������ʹƽ����������������ķ����ƶ���3�֣�
�ڽ������������������ʹƽ����������������ķ����ƶ���3�֣�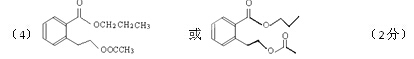
������������������п�֪��C�ܸ�NaHCO3������Ӧ������C�����Ȼ����ٴ�C��D�ķ�Ӧ�������Կ���������һ��������Ӧ�����DΪ��������Ϊ�ú�D����Է���������ȣ�����D���ִ�Ϊ��C��һ��̼ԭ�ӵĴ�����EΪ��֧���Ļ�������������ʹ�����ֱ���ġ�
E�е�̼ԭ����ĿΪ��
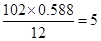 ����ԭ����Ϊ��
����ԭ����Ϊ��
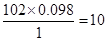 �����ʽΪ��C5H10O2
�����ʽΪ��C5H10O2
Ҳ����˵EΪ������������ǿɵ����½⣺
��1���Ȼ� e
��2��CH3COOH+CH3CH2CH2OH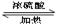 CH3COOCH2CH2CH3+H2O��2�֣�
CH3COOCH2CH2CH3+H2O��2�֣�
��3���ټӿ췴Ӧ����
�ڽ������������������ʹƽ����������������ķ����ƶ���3�֣�

���㣺�����л���֮����ת����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪ijһ��ȼ�Ϻ�̼�����������Ԫ�أ�Ϊ�ⶨ��ȼ�ϵ���ɣ�����ȼ�Ϸ��뵽����������ȼ�գ���ʹ������CO2��H2O�����Լ�ʣ���O2ȫ��ͨ������ͼ��ʾ��װ�ã��õ����±����г���ʵ�����ݣ��������ɵ�������ȫ�������գ���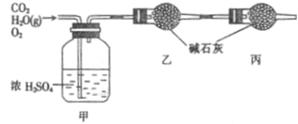
| | ʵ��ǰ | ʵ��� |
| ������ / g | 101��1 | 103��8 |
| �ҵ����� / g | 82��0 | 86��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��18�֣��ӱ������еõ�һ����A��C10H16�����Ш�������������A��ط�Ӧ���£�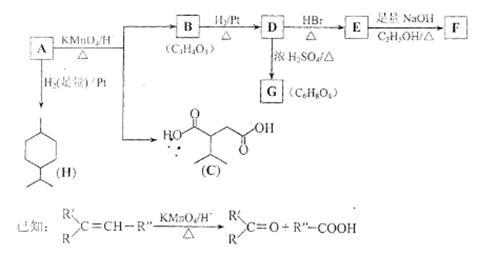
��1��H�ķ���ʽΪ ��
��2��B���������ŵ�����Ϊ ��
��3����������COOCH3���ŵ�C��ͬ���칹�干�� �֣������������칹�������к˴Ź������׳���2�����շ���칹��ṹ��ʽΪ ��
��4��B��D��D��E�ķ�Ӧ���ͷֱ�Ϊ �� ��
��5��GΪ����Ԫ���Ļ����д����ṹ��ʽ�� ��
��6��F��һ�������·����ۺϷ�Ӧ�ɵõ�һ�ָ���ˮ����֬������֬����Ϊ ��
��7��д��E��F�Ļ�ѧ����ʽ�� ��
��8��A�Ľṹ��ʽΪ ��A������ʵ�����Br2���мӳɷ�Ӧ�IJ��ﹲ�� �֣������������칹����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�
���������������ҩ�Ϳ�ϵ�����Ӧ�ù㷺��
��1�����й��ڻ�����I��˵������ȷ���� 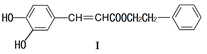
| A����FeCl3��Һ��������ɫ |
| B�����ܷ���������Ӧ��������Ӧ |
| C�������巢��ȡ���ͼӳɷ�Ӧ |
| D��1mol������I�����2molNaOH��Ӧ |

 �������Ʊ�Ϳ�ϡ��䵥��Ľṹ��ʽΪ ���������Ʒ�Ӧ�ٵķ���������ϩΪ�л�ԭ�Ϻϳɸõ��壬�漰�ķ�Ӧ����ʽΪ ��
�������Ʊ�Ϳ�ϡ��䵥��Ľṹ��ʽΪ ���������Ʒ�Ӧ�ٵķ���������ϩΪ�л�ԭ�Ϻϳɸõ��壬�漰�ķ�Ӧ����ʽΪ �� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�������з�Ӧʽ����˵����Ӧ���͡�
(1)��ϩͨ����ˮ��
(2)2����ϩ�ڴ������������ɾ�2����ϩ
(3)�ɱ��������� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ�Cѡ��5���л���ѧ������(15��)
�ҹ����ϡ������ȵ�ʢ����ɽ������������ȩ�����ܸߣ����������ɴﵽ60%��90%������ȩҲ�������������ϩΪԭ���˹��ϳɣ�����ȩ�ֿ������ϳ�������ͪ���㾫���ϣ���ϳ�·�����£� 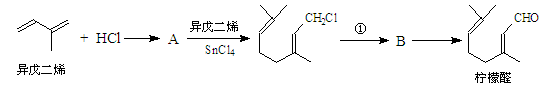

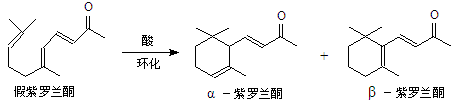
��֪����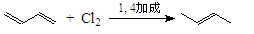
��
��ͬһ̼ԭ����������˫���ṹ���ȶ���
�Ը�������ת����ϵ�ش��������⣺
��1��д��A�Ľṹ��ʽ ��C�Ľṹ��ʽ ��
��2���ٶ�Ӧ�ķ�Ӧ������ ����Ӧ�۵ķ�Ӧ������ ��
��3��д��Bת��Ϊ����ȩ�Ļ�ѧ����ʽ ��
��4�����ݷ�Ӧ�ڵķ�Ӧ����д��CH3CHO��������HCHO��Ӧ����Ľṹ��ʽ��
��
��5����������ȩ�к���̼̼˫����ʵ�鷽���ǣ� ��
 ��6������������ͪ���£�������ͪ�кܶ�ͬ���칹�壬����������������ͬ���칹���� �֡�
��6������������ͪ���£�������ͪ�кܶ�ͬ���칹�壬����������������ͬ���칹���� �֡�
�ٺ���һ������ �����ڴ����Ҳ��ܷ�����������Ӧ
�ۺ˴Ź���������ʾ��5����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(12��)����ʽΪC3H6O3�������ж���ͬ���칹�壬��д����������Ҫ��ĸ���ͬ���칹��Ľṹ��ʽ(������ͬһ̼ԭ�����������ǻ�)�����ش�������⣺
��1��������û�м�����1 mol������������Na��Ӧ����1 mol H2��������NaHCO3��Һ��Ӧ����Ľṹ��ʽΪ����������������������������������ˮ(��ѡ����ס�)�� ԭ�������������������������������ʵ��۵����Ը�����Է���������ӽ������������۵㣬ԭ������������������������������
��2���ҷ�����̼�����ֱ������ֻ�ѧ��������Ļ�ѧ������ͬ�����������Na����Ӧ�����ҽṹ��ʽΪ�������������������������������������������������(���������ȩ���������ᡱ������)������Һ���ܶ�Ӧ��ˮ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣����и����е������л����������ͬ�����ʡ�ͬϵ���ͬ���칹��ȣ����ж�����֮��Ĺ�ϵ
��1��2��������Ͷ��� ________________
��2���������2��2����������___________________
��3������ױ����ұ�_____________________
��4��1����ϩ�ͻ�����_________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)����ʽΪC7H8O�ķ����廯�����У���FeCl3��Һ��Ϻ�����ɫ�Ͳ�����ɫ�����ʷֱ���3�ֺ�2�֣���д�����е�һ�֣�д���ṹ��ʽ���� �� ��
�ٳ�ѧ����2������˵�����㻯��������Ա�����Ӱ�죬�û�ѧ����ʽ��ʾ �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com