
����Ŀ��Ŀǰȫ��Χ�ڻ�����Ⱦ���ܹ�ע������������Ӧ������������ɫ��ѧ������ԭ�Ӿ������������ҵ�����еķ���������Ϊ��Ҫ����ҵ������������ʱ����ͬʱ������һ�ֳ�������Ҫ������������Ư����������(NaClO2)���乤���������£�
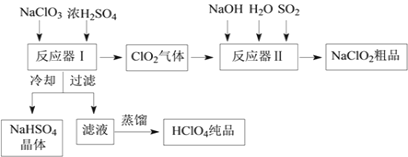
��֪����NaHSO4�ܽ�����¶ȵ����߶������ʵ������¿ɽᾧ�������ڸ�����������Ϊֹ������֪���е���ǿ�ᣬ�е�90������ش��������⣺
��1����Ӧ�����з�����Ӧ�Ļ�ѧ����ʽΪ_______����ȴ��Ŀ����____________��
��2����Ӧ�����з�����Ӧ�����ӷ���ʽΪ__________________________��
��3��ͨ�뷴Ӧ�����е�SO2��H2O2����ͬ��������NaClO2�����Ҫ˵��˫��ˮ�ڷ�Ӧ���ܴ���SO2��ԭ����_________________________��
��4��Ca(ClO)2��ClO2��NaClO2�Ⱥ��Ȼ����ﶼ�dz��õ���������Ư������д����ҵ������������ʯ������Ư�۾��Ļ�ѧ����ʽ��___________________________________��
���𰸡� 3NaClO3+3H2SO4��HClO4+2ClO2��+3NaHSO4+H2O ����NaHSO4���ܽ�ȣ�ʹNaHSO4�ᾧ���� 2ClO2+SO2+4OH����2ClO2��+SO42��+2H2O H2O2�л�ԭ��Ҳ�ܰ�ClO2��ԭΪNaClO2 2Cl2+2 Ca(OH )2��CaCl2+ Ca(ClO)2+2H2O
����������1�����ݼ��뵽��Ӧ�����е�ԭ����NaClO3��Ũ���ᣬ��������ClO2���塢NaHSO4��HClO4��Һ��������д��ѧ����ʽ�Ĺ���������ѧ��Ӧʱ����ԭ�ӵ��������Ŀ���䣬��д����ѧ��Ӧ����ʽΪ��3NaClO3+3H2SO4=HClO4+2ClO2+3NaHSO4+H2O��Ȼ��Ӧ���������Һ��ȴ�õ�NaHSO4���壬˵���������Ƶ��ܽ�����¶ȵĽ��Ͷ���С��
��2������ͨ�뵽��Ӧ�������еķ�Ӧ����NaOH��SO2��H2O��ClO2����������NaClO2���ȵĻ��ϼ���+4�۱����+3�ۣ��õ����ӣ�����������ԭ��Ӧ��ԭ����Ӧʧ���ӣ���+4�۱��+6�ۣ�����������ԭ��Ӧԭ�����������õ������������ڻ�ԭ��ʧȥ���ӵ���������������ԭ��Ӧ����ʽΪ2ClO2+SO2+4OH-=2ClO2-+SO42-+2H2O��
��3���������Ӧ�����е�SO2��H2O2����ͬ��������NaClO2��˵���任���ʺ�������ԭ��Ӧ�����ܷ�������NaClO2��Ҳ��˵��H2O2Ҳ���л�ԭ�ԣ�Ҳ�ܰ�ClO2��ԭΪNaClO2��
��4����������Ư��������ԭ����Ư��ԭ�����������ǵ�ǿ���������ﵽĿ�ģ����ݽ̲�֪ʶ��ҵ����ȡƯ��������������ʯ�ҷ�Ӧ�õ��ģ��䷴Ӧԭ��Ϊ2Cl2+2 Ca(OH )2��CaCl2+ Ca(ClO)2+2H2O��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F�Ƕ�����Ԫ�أ����ڱ���A��B��B��C���ڣ�C��Eͬ���壻A��C����������֮��Ϊ2:3��B��������������C��������������1���� FԪ�ص�ԭ�������ڱ��а뾶��С������������D2C2��ˮ��Ӧ����C�ĵ��ʣ�����Һʹ��̪��Һ��졣
��1��F�����ֺ��ر�ʾ����Ϊ______��E������Ϊ______��D������������ˮ����ĵ���ʽ��______��
��2����B��C��D��E��Fԭ���γɵĵ������������Ӱ뾶��С����____�����Ӱ뾶������____���������ӷ��ţ�
��3��A��B��C���⻯���ȶ���˳��Ϊ_____���÷���ʽ��ʾ����B���⻯���B������������ˮ���ﷴӦ����Z,��Ӧ�ж��ѻ�ѧ����_____���γɵĻ�ѧ����________��������Ӽ����������Լ��������Ǽ��Լ��������������
��4��F2C��F2E�У��е�ϸߵ���__________���ѧʽ��������Ҫԭ����_________��
��5�����־���C��D��E��F����Ԫ�صĻ��������Ӧ�ų�����ķ�Ӧ���ӷ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ڳ���Ԫ�ص���
A�� ͭ���� B�� п���� C�������� D�� �⡢��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ������������ԭ��Ӧ�����������ȷ�Ӧ����(����)
A. þ���ڿ�����ȼ�� B. Ba(OH)2��8H2O��NH4Cl�ķ�Ӧ
C. ��Ƭ��ϡ����ķ�Ӧ D. ���ȵ�̿��CO2�ķ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������п����100mLl8��4mol/LŨ�����м��ȣ���ַ�Ӧ��п��ȫ�ܽ⣬ͬʱ��������X 22��4L(��״��)������Ӧ�����Һϡ����0��8L�������Һc(H+)=0.1mol/L���������жϴ������
A. ����XΪSO2��H2�Ļ���� B. ����X��SO2��H2�������� C. ��Ӧ�й�ת��2mol���� D. ��Ӧ�й�����Zn 65 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ƣ�NaClO2������Ҫ��Ư����ijС�鿪չ����ʵ�飬����ͼװ����ȡ��ˮNaClO2���壬�ش��������⣺
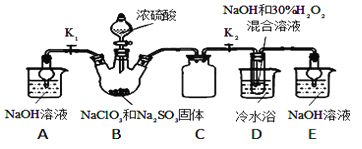
��֪��NaClO2������Һ�ڵ���38��ʱ����NaClO23H2O������38��ʱ����NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��
��1��װ��C��������_________________��
��2����֪װ��B�еIJ�����ClO2���壬��B�з�Ӧ�ķ���ʽΪ_________________��װ��D�з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ_________________________��
��3����װ��D��Ӧ�����Һ�л����ˮNaClO2����IJ�������Ϊ������ѹ��55�������ᾧ����____________������38����60����ˮ________������_____________�����¸���õ���Ʒ��
��4�������ȥD�е���ˮԡ�����ܵ��²�Ʒ�л��е�������_________��____________��
��5���ⶨ��Ʒ��NaClO2�Ĵ��ȣ��ⶨʱ��������ʵ�飺
ȷ��һ����������Ʒ��������������ˮ������KI���壬�����������·������·�Ӧ��ClO2��+4I��+4H+�T2H2O+2I2+Cl���������û��Һϡ�ͳ�100mL������Һ��ȡ25.00mL������Һ�����������Һ��ָʾ������c molL��1 Na2S2O3��Һ�ζ����յ㣬������ı���Һ�����ƽ��ֵΪV mL����֪��I2+2S2O32���T2I��+S4O62���������������ȡ����Ʒ��NaClO2�����ʵ���Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ֪ʶ����������ϢϢ��ء�
I.�輰�仯����
��1���輰�仯�������ִ���Ϣ������Ӧ�ù㷺������������������оƬ�IJ�����__________��д��ѧʽ��������������ά�IJ�����__________��д��ѧʽ�������ά�ڼ����������ױ���ʴ����д����ص����ӷ�Ӧ����ʽ______________________
II.�ȼ��仯����
��2��Ư�����������Ʒ������Ҫ�ɷ���__________________��д��ѧʽ����Ư�۱��治���ױ��ʣ���д��Ư��ʧЧ�ķ���ʽ________________���粻С�İ�Ư�������飨��Ҫ�ɷ�Ϊ���ᣩ��ϣ�����������ʹ���ж�����д���йط�Ӧ�����ӷ���ʽ________________________��
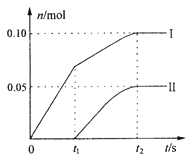
��3��ijʵ��С�飬��һ������ʯ�����л������ٵ�ͨ���������������ֲ����л�������ClO3�����������ۺ���Ϊ���Ƿ�Ӧ�����¶����ߵ�ԭ��������ClO����ClO3���������ӵ����ʵ�����n���뷴Ӧʱ��(t)�Ĺ�ϵ���ߣ����Ա�ʾΪ��ͼ��������������ˮ�ķ�Ӧ����
��ͼ������I��ʾ_____________���ӵ����ʵ����淴Ӧʱ��仯�Ĺ�ϵ��
����ȡʯ�����к���Ca(OH)2�����ʵ���Ϊ__________mol��
����ȡһ����ڵ����ʵ�����ʯ���飬�Խϴ������ͨ��������������Ӧ���ò�����Cl�������ʵ���Ϊ0.37mol���������n(ClO��)/n(ClO3��)=______________��
III.�����仯����
��4�����������������ŷų����ķ�������NO��NO2�ȴ�����Ⱦ�
�ٹ�ҵ�ϳ���ԭ����NOx+NH3��N2+H2O��ʹ��ת��Ϊ����N2������NO��NO2�Ļ��3.0L������3.5L��ͬ״����NH3��ȫ��Ӧ��ȫ��ת��ΪN2������ԭ���������NO��NO2�����ʵ���֮����_____________��
����֪���ܳ�ȥ����β����2NO2+2NaOH��NaNO2+NaNO3+H2O;NO+NO2+2NaOH��2NaNO2+H2O
��������β�������ķ�Ӧԭ�������������в��ܱ�����NaOH��Һ���յ���_______________
A��1molO2��4molNO2��
B��1molO2��4molNO��
C��1molNO��5molNO2�� ��
D��4molNO��1molNO2
��5�������ŷŵ�β�������е������������Ⱦ��������ɲ����������������Ҫԭ����_____________
A��ȼ�պ���������ȼ�ϡ�������������
B��ȼ�պ�Ǧ����
C������ȼ�ղ���֡�����������������
D����ȼ���п����е�N2�ڸ����±�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ������ ��
A. H2SO4����ˮ�ܵ����H����SO42�����������������ӻ�����
B. Ba(OH)2�����мȴ��ڹ��ۼ��������Ӽ�
C. SiO2����ԭ�Ӿ��壬�ۻ��ƻ����ۼ��ͷ��Ӽ�������
D. �ɱ�����ʱ�������ڹ��ۼ��ᷢ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧΪ�˼���Ũ������ľ̿���ڼ��������·�Ӧ����������������ѡ��������ͼ��ʾ��ʵ��װ�á�
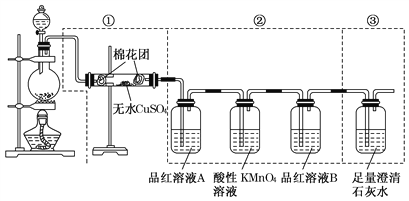
(1)д��Ũ������ľ̿���ڼ��������µĻ�ѧ����ʽ______________________��
(2)������ˮ����ͭ��������___________________________��
(3)��������KMnO4��Һ��������__________________________��
(4)���������õ�Ʒ����Һ�����ǵ����÷ֱ���
A_____________________�� B ___________________ ��
(5)���г��ֵ�����____________________________________________________��
������Ӧ�����ӷ���ʽ_________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com