
����Ϊһ��������Դ���㷺�������ã��������������ص�����˵���У�����Ϊ����ȷ���� (����)��
A����ȼ�ղ���Ի�����������Ⱦ�����õ���Ч����
B����Si(s)��O2(g) SiO2(s)����H����858.6 kJ��mol��1��֪����ȼ�շų���������
SiO2(s)����H����858.6 kJ��mol��1��֪����ȼ�շų���������
C����Ȼ���й�Ĵ����ḻ�����Դ���Ȼ��ֱ�ӻ�õ��ʹ�
D����������䡢���棬�Ӱ�ȫ�Ƕȿ��ǣ�������ѵ�ȼ��֮һ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����
A��������̼������Һ��Ӧ��
2H+��CO32��== CO2����H2O
B��������Һ������������ͭ��Ӧ��
CH3COOH��OH�� �� CH3COO����H2O
C����������Һ��ͨ������������̼��
2C6H5O����CO2��H2O �� 2C6H5OH��CO32��
D����ȩ��Һ��������������Һ���ȣ�
HCHO+4[Ag(NH3)2]++4OH-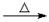 CO32��+2NH4++4Ag��+6NH3+2H2O
CO32��+2NH4++4Ag��+6NH3+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����ˮ�к���Zn2����Hg2����Fe3����Cu2��4�������ӡ��ס��ҡ���3λͬѧ��ƵĴӸ���ˮ�л��ս���ͭ�ķ������¡�
�ף�
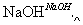

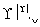
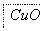
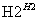
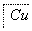
�ң�
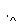


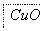
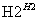
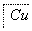
����
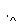

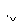


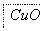
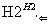
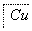
�����ж���ȷ���� (����)��
A������ʵ�鷽���ж����Ƶô�����ͭ
B���ҷ����мӹ������ۿ��Խ�4��������ȫ����ԭ
C�������еķ�Ӧ�漰�û����ֽ⡢���ϡ����ֽ�4�ַ�Ӧ����
D�������������������Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ν�Ͻ𣬾��Dz�ͬ�ֽ���(Ҳ����һЩ�ǽ���)������״̬���γɵ�һ���ۺ�������������ֽ������ۡ��е㣬�ж����в����γɺϽ���� (����)��
| Na | Cu | Al | Fe | |
| �۵�/�� | 97.5 | 1 083 | 660 | 1 535 |
| �е�/�� | 883 | 2 595 | 2 200 | 3 000 |
A��Cu��Al B��Fe��Cu
 C��Fe��Na D��Al��Na
C��Fe��Na D��Al��Na
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ɻ������ױ��Ǻš���ը����������ĺ���Ұ���ר���Ʋ⡰���ױ��ǡ�����ɻ���ʧ��ԭ��֮һ�Ǹ����ں���ɻ�����һƬ������ĸ��������룬����ɻ���������¶�Ѹ�����߶��ۻ����塣
(1)������Ƭ��ʾ���ڡ����ױ��Ǻš������·��м�ƬС��Ƭ���䣬�����˿�ѧ�ҵ�ע�⣬����һ�ָ����ں���ɻ������������մ�Ƭ������Ҫ������_________________________________________________________________��
A�����Ӻ���ɻ���ǿ�ȣ���ֹ���Ǻ�̫������ײ��������ɻ�
B����̫����ת��Ϊ���ܹ�����ɻ�ʹ��
C�������״���٣����ܵ���ָ�����ĵ�ָ��
D���ڷ��ش�����ʱ���մ� ���¸��ȣ���Ч�ر�������ɻ�ƽ�����ص���
���¸��ȣ���Ч�ر�������ɻ�ƽ�����ص���
(2)��������һ�ֽ����մɣ������մ������մɺ��������ɵķ����ʵĸ��ϲ��ϡ��մ���Ҫ��Al2O3��ZrO2������������������Ҫ��Cr��Mo��W��Ti�ȸ��۵���������й��ڸ��ϲ��ϵ�˵������ȷ����________��
A�������ֻ��������Ͻ���(�������ǽ���)�ۺ϶��ɵ����ʽи��ϲ���
B�����ϲ���һ�����ǿ�� �ߡ������ᡢ���¡���ʴ���������ܣ����ۺ� �����ϳ����˵�һ����
�ߡ������ᡢ���¡���ʴ���������ܣ����ۺ� �����ϳ����˵�һ����
C�����������Բ�����ά����֬��ɵĸ��ϲ��ϣ����������塢���������ȣ�Ҳ����ӡˢ��·��
D�����ϲ��ϵ��ۡ��е�һ������ĸ���ɳɷֵ��ۡ��е��
(3)���մɺ��������ĥ����Ͼ��ȣ����ͺ��ڲ������������սᣬ�Ϳ��Ƶý����մɡ������մɼ��н������մɵ��ŵ㣬���ܶ�С��Ӳ�ȸߡ���ĥ�������Ժã�������Ϊ��������ȶ����ѡ�ʵ������һ�����մ��Ƴɵ�������������ʢ��������Щ����________��
A��NaOH��Һ B��KNO3��Һ
C������� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�ֹ裬����������ȡ����������Ʒ�ֱ�Ͷ��������ϡ�����������ϡ����������Һ�У��ų�������H2����ôֹ�������Ĺ�ϵ��ȷ����(��ʾ��Si��2NaOH��H2O===Na2SiO3��2H2��) (����)��
A�����ʵ���֮��Ϊ��1��1 B�����ʵ���֮��Ϊ1��2
C������֮��Ϊ4��1 D������֮��Ϊ2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ټ���ƿ ����Ͳ ���Թ� ������ �������� ������ƿ ����ƿ�����þƾ��Ƽ��ȵ���
A.�ڢۢ� B.�ۢޢ� C.�٢ۢ� D.�ۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ͬ��������һ��ʢ��NH3����һ��ʢ��N2��O2����ͬ�¡�ͬѹ�£��������ڵ�����һ��������ͬ��
A��ԭ���������� B. ������������ C. ���������� ��D.����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F��G���ֶ���������Ԫ�ص�ԭ��������������A��E������������ͬ������������Ԫ�ص�ԭ���У�Eԭ�ӵİ뾶���B��C��F�����ڱ����� �ڣ�B��Cͬ���ڣ�C��Fͬ���壬Fԭ�ӵ���������Cԭ����������2����A��C���γ����ֳ�����Һ̬������X��Y(��Է�������X<Y)��D�γɵķ���Ϊ˫ԭ�ӷ��ӡ��ش����⣺
(1)DԪ�ص�����Ϊ________��F�γɵ����ӵĽṹʾ��ͼΪ _________________________________________________________________ ___��
(2)д��Һ̬������Y��һ����;_____________________________________________
____________________________________________________ ____________________��
____________________��
(3)��ij�ֽ�����������A��C��E��ɵĻ������ˮ��Һ��Ӧ������������ɳ����� ����д���÷�Ӧ�����ӷ���ʽ______________________________________��
ʹ������������ڵ�������______________________________________________
________________________________________________________________________��
(4)P��Q�������ʶ�����A��C��E��F����Ԫ����ɵ��Σ���ˮ��Һ�������ԣ������� ������P��Qǡ����ȫ��Ӧ��д���÷�Ӧ�����ӷ���ʽ�� ________________________________________________________________________
________________________________________________________________________��
(5)A��B��G����Ԫ����������γɵĻ������ڿ����������γɰ��̣���Ӧ�Ļ�ѧ���� ʽΪ
_______________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com