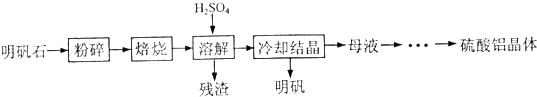
��2012?�Ͼ�ģ�⣩ʵ����������李�����ͷ���м�Ʊ���������茶���ķ������£�
����1��������м�������ȵ�̼������Һ������ˮϴ�ӣ�
����2����ʢ�нྻ��м���ձ��м���ϡH
2SO
4��Һ��ˮԡ���ȣ�ʹ��м��ϡ���ᷴӦ����������ð������Ϊֹ�����ȹ��ˣ�
����3������Һ�м���һ�����ģ�NH
4��
2SO
4���壮
��1 �����������塢����李���������茶�����ܽ�ȣ�g/100g H
2O��
| ���� |
0�� |
10�� |
20�� |
30�� |
40�� |
50�� |
60�� |
| FeSO4?7H2O |
15.6 |
20.5 |
26.5 |
32.9 |
40.2 |
48.6 |
- |
| ��NH4��2SO4 |
70.6 |
73.0 |
75.4 |
78.0 |
81.0 |
- |
88.0 |
| ��NH4��2SO4?FeSO4?6H2O |
12.5 |
17.2 |
21.0 |
28.1 |
33.0 |
40.0 |
44.6 |
��1������2�п������ɵ����������в����������ķ�����
����������
����������
��
��2����0��60�淶Χ�ڣ�����������������淋Ļ����Һ�пɻ����������茶����ԭ����
��0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С
��0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С
��
��3��Ϊ�˴Ӳ���3������Һ�л����������茶��壬������
����Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�����
����Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�����
��
��4���ⶨ��������茶�����Fe
2+�����IJ������£�
����1��ȷ��ȡ��������茶�����Ʒa g��ԼΪ0.5g���������Ƴ�100mL��Һ��
����2��ȷ��ȡ25.0mL�����������Һ��250mL��ƿ�У�
����3��������Ũ��ԼΪ0.1mol?L
-1KMnO
4��Һ�ζ�����Һ���ȶ��ķۺ�ɫ����Ϊ�յ㣻
����4����ʵ�鲽��1��3�ظ�2�Σ�
�ٲ���1������100mL��Һ��Ҫ�IJ���������
��������100mL����ƿ����ͷ�ιܡ��ձ�
��������100mL����ƿ����ͷ�ιܡ��ձ�
��
��Ϊ�����Ʒ��Fe
2+�ĺ��������貹���ʵ����
ȷ�ⶨ�ζ������ĵĸ��������Һ�����
ȷ�ⶨ�ζ������ĵĸ��������Һ�����
��



 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�