
����Ŀ��ij����Na2O���ʵ�Na2O2������һ����ѧʵ��С������H2O��Na2O2�ķ�Ӧ���ⶨ����Ʒ�Ĵ��ȡ��ɹ�ѡ���װ�����£�

��ش��������⣺
��1��װ�â�������a��������______��
��2��������װ�ÿ�����װһ����IJⶨ����������Ʒ���ȵ�ʵ��װ�á�
��.��ʵ��װ�õ������________(����ĸ)��
a. �٢ܢ� b. �٢ۢ� c. �ڢܢ� d. �ۢݢ�
��.��ѡ��װ�õ�����˳��Ӧ��__________(����ӿڵ���ĸ�����ӽ���ʡ��)��
��3��д��ʵ����Na2O2������Ӧ�Ļ�ѧ����ʽ_____________��
��4��������ʵ���������Һ���Ƴ�Ũ��Ϊ1.0mol/L����Һ���ش��������⡣
����400mL����Һ��ͨ��0.3mol CO2����������Һ��HCO3�C��CO32�C�����ʵ���Ũ��֮��ԼΪ___________��
A. 1:3���� B. 1:2������C. 2:1��������D. 3:1
����������Һ�����ᾧ�õ�Na2CO3��NaHCO3�����������ѡ���в���ȷ�ⶨ�������Na2CO3������������____________��
a. ȡa g������ּ��ȣ��ڸ���������ȴ�����£�����b g
b. ȡa g�����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b g����
c. ȡa g�����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����b g
d. ȡa g�����������Ba(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b g����
���𰸡���Һ©�� A G��A��B��F 2Na2O2+2H2O=4NaOH+O2�� C c
��������
��װ����������a�������Ƿ�Һ©����
��������Ϊ��Ӧװ�ã�ͨ���ų�����ˮ������������еõ�ˮ������������������ƴ��ȣ�
��ʵ����Na2O2������Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2����
��HCO3�C��CO32�C�����ʵ����ֱ�Ϊx��y���������Ĺ�ϵ���м��㡣
��װ����������a�������Ƿ�Һ©����
��������Ϊ��Ӧװ�ã�ͨ���ų�����ˮ������������еõ�ˮ����������װ���DZȽϼIJⶨ����������Ʒ���ȵ�ʵ��װ�ã����A��ȷ��ѡ��װ�õ�����˳��Ӧ��G��A��B��F��
��ʵ����Na2O2������Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2����
����HCO3�C��CO32�C�����ʵ����ֱ�Ϊx��y����400mL����Һ�����ʵ���n=1.0mol/L��0.4L=0.4mol��ͨ��0.3mol CO2��x+y=0.3��x+2y=0.4�����x=0.2mol��y=0.1mol��Ũ��֮�ȵ������ʵ���֮�ȣ����������Һ��HCO3�C��CO32�C�����ʵ���Ũ��֮��ԼΪ2:1��C��ȷ��
��aѡ�ȡa g������ּ��ȣ��ڸ���������ȴ�����£�����b g�����ٵ�����Ϊ̼�����Ʒֽ����ɵĶ�����̼��ˮ������������ܼ����̼���Ƶ�������������a���������⣻
bѡ�ȡa g�����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b g���壬���õ��Ȼ��ƹ��壬���ݷ����������̼���Ƶ�������������b���������⣻
cѡ�ȡa g�����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����b g����ʯ�����ӵ������Ƕ�����̼��ˮ����������ˮ�����������㣬�ʲ��ܼ���̼���Ƶ�������������c�������⣻
dѡ�ȡa g�����������Ba(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b g���壬�õ�̼�ᱵ���������ݷ����������̼���Ƶ�������������d���������⣻
�����������𰸰�Ϊc��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�������������������������з�����ȡ�ģ�MnO2��4HCl(Ũ) ![]() MnCl2��Cl2����2H2O���Իش��������⣺
MnCl2��Cl2����2H2O���Իش��������⣺
(1)�÷�Ӧ��������ԭ��Ӧ��__________(����������������)�������������ԭ��Ӧ����ָ��__________����������______________�ǻ�ԭ����____________���������______________�ǻ�ԭ�����������������ԭ��Ӧ�ж������ԣ�__________��__________����ԭ�ԣ�__________��____________��
(2)д���÷�Ӧ�����ӷ���ʽ______________________________��
(3)��˫���ŷ�����÷�Ӧ����ת�Ƶķ������Ŀ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Һ�����ԭ��ȥ��ˮ����![]() ��һ�����ǰ���ļ�����ij����С���о��÷�����ʹ�õĹ������Pd-Cu/TiO2���Ʊ��ʹ�������������¡�
��һ�����ǰ���ļ�����ij����С���о��÷�����ʹ�õĹ������Pd-Cu/TiO2���Ʊ��ʹ�������������¡�
���Ʊ�Pd-Cu/TiO2�ķ�Ӧ��������ͼ��ʾ������ʹTiO2������ɷ��룬������Pd������TiO2���档�������Ʒ�������Cu���õ���TiO2������������ܽӴ���Pd-Cu���״ء�
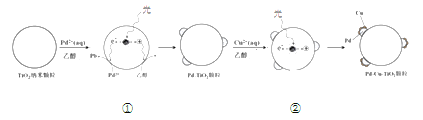
��1���ù����У��Ҵ�����__________������������������ԭ������Ӧ��
��2�����ڢ��в�ȫ�����������γ�Pd-Cu���״صķ�Ӧ����ʾ��ͼ_______��
��Pd-Cu/TiO2�ֲ�����ԭ������ͼa��ʾ������������ͬ����ͬpHʱ����Ӧ1Сʱ��![]() ת���ʺͲ�ͬ�������ܻ�ԭ��������ռ�����ʵ����İٷֱ���ͼb��ʾ��
ת���ʺͲ�ͬ�������ܻ�ԭ��������ռ�����ʵ����İٷֱ���ͼb��ʾ��
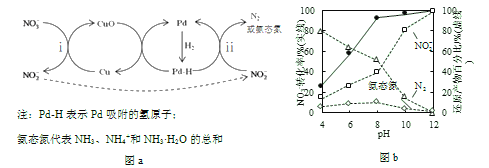
��3����Һ�����ԭ�������õĻ�ԭ����__________��
��4��i�����ӷ���ʽ��__________��
��5���о�������OH�C��Pd������![]() �����������ή��Pd����
�����������ή��Pd����![]() ������������Pd����H������Ӱ�첻��
������������Pd����H������Ӱ�첻��
����pH����N2�Ͱ�̬���ڻ�ԭ�����еİٷֱȾ���С��ԭ����____________�����·�Ӧ���Ļ�ѧ��Ӧ���ʽ��͡�
����pH��С����ԭ������![]() �ı仯������__________��˵��Pd����������
�ı仯������__________��˵��Pd����������![]() ��H�ĸ����ȱ�Է�Ӧ__________�������ӷ���ʽ��ʾ����������
��H�ĸ����ȱ�Է�Ӧ__________�������ӷ���ʽ��ʾ����������
��7��ʹ��Pd-Cu/TiO2��ͨ��������ҺpH���ɽ�![]() �����ܶ��ת��ΪN2�����巽����__________��
�����ܶ��ת��ΪN2�����巽����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ�������������Ӧʵ�����
A.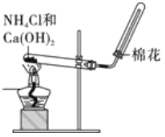 ʵ������ȡ���ռ�NH3
ʵ������ȡ���ռ�NH3
B.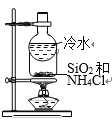 ����SiO2��NH4Cl
����SiO2��NH4Cl
C. ������ᾧ�����Ƿ��нᾧˮ
������ᾧ�����Ƿ��нᾧˮ
D.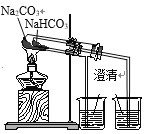 ��֤���ȶ��ԣ�Na2CO3>NaHCO3
��֤���ȶ��ԣ�Na2CO3>NaHCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ú���ۺ����ñ��ܹ�ע�������µ�ת����ϵ��CO��H2��ͬ�������Էֱ�ϳ�A��B����֪��A������������ܶ���14��B�ܷ���������Ӧ��CΪ��������ζ����

��1���л���C�к��еĹ����ŵ�������____________________��
��2����Ӧ�ݵķ�Ӧ����Ϊ______________��
��3��д���۵ķ�Ӧ����ʽ ____________��
��4������˵����ȷ����______________��������ĸ��
A���ڢٲ���ú��Һ����Ϊú���ۺ����õ�һ�ַ���
B���л���B��C������������������ͭ������Ӧ
C���л���C��D��ˮ��Һ������ɱ����������
D�������������л���D�����ķ��룬����������������Һ�����÷�Һ�ķ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij�Ͻ��ĩ�������⣬����������ͭ�е�һ�ֻ����֣�ij��ȤС������ʦ��ָ���£��ԺϽ�������ͭ�Ĵ����������������̽����
���������ϣ�����ͭ��������������Һ��Ӧ��
�����룩����1���úϽ��ĩ�У��������⣬����������
����2���úϽ��ĩ�У��������⣬������ͭ��
����3���úϽ��ĩ�У��������⣬������_________�������ƣ���
��ʵ��̽��������ʵ�����ѡ����Լ��ǣ�10%�����ᡢ30%������������Һ��
ʵ�鷽�� | ʵ������ | ���� |
��ȡһ�����ĺϽ��ĩ���ӹ�����____����ַ�Ӧ����ˣ��������á� | ��ĩ�����ܽ⣬��������ų��� | �Ͻ���һ���������� |
��ȡ����������������ӹ�����______����ַ�Ӧ�� | ������ĩ�����ܽ⣬��������ų�����Һ����dz��ɫ�� | �Ͻ���һ������______�� |
��̽�����ۣ�����3������
����˼��һ����˵�����ý�������������ᷴӦ���������ᡢ��ܷ�Ӧ��˵����������������ʡ�д����������������Һ��Ӧ�����ӷ���ʽ__________��
��֪ʶ���죩��һ�������£�������������ˮ��Ӧ��д������Ӧ�����£�����ˮ������Ӧ�Ļ�ѧ����ʽ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵������ȷ����
A.���������������Դ��̫���ܣ�����Ȼ������̫������ɹ�����ֲ��Ĺ������
B.��ֲ��Ľոѵȼ����������ͳ��У��ڸ��������£���������ϸ�������ã���������
C.ˮú���������ϳɿ��Եõ��״���ȼ��
D.����Ͻ��Ǽ���������������ͨ����ѧ��Ӧ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�����������������ȷ���ǣ� ��
A.��״���£�������ΪNA��CO��N2����������ԼΪ22.4L������Ϊ28g
B.25�桢101.3kPaʱ��11.2LH2�к��е�ԭ����ΪNA
C.10 mL��������Ϊ98%��H2SO4����ˮϡ����100mL��H2SO4����������Ϊ9.8%
D.1mol/L Na2SO4��Һ����������Ϊ3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���������Լ��Ʊ���Ӧ�ù㷺��ij�о�С�����������ַ����Ʊ�FeCO3����֪�� FeCO3 �ǰ�ɫ���壬������ˮ��
I���о�С�������������������Ʊ�FeCO3����������Ҫ����K2Fe6(SO4)4(OH)12��ZnO��Fe2O3��������CaO��MgO�� SiO2 �ȡ�
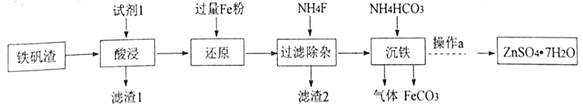
��1���Լ�1�ǹ�����_______������ 2�ijɷ���__________ (д��ѧʽ)��
��2����������������Ӧ�����ӷ���ʽ��____________��
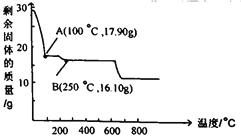
��3�����������Һ��������a�����پ��������ˡ� ϴ�ӡ�����õ�𩷯(ZnSO4��7H2O)��������a����__��ȡ28.70g𩷯(ZnSO4��7H2O)���ȣ�ʣ�����������仯��ͼ��100��Cʱ���ù���Ļ�ѧʽ____��
II���о�С���ֲ�������װ����ȡFeCO3 (C�г�װ����)�������Ĺؼ�����Na2CO3��Һ(pH=12.11)ͨ��һ��ʱ��CO2����ҺpHΪ7���ٵμ�һ����FeSO4��Һ��������ɫ������
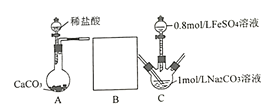
��1������װ��B______________��B�������Լ�Ϊ______________��
��2������1mol/L Na2CO3��Һ��ͨ��CO2��Ŀ����_________________________��
��3��FeCO3��������[CH3CH(OH)COOH]���Ƶÿ���������������Ѫ������ʵ��С����KMnO4�ⶨ��Ѫ�����������������������������������������������������������������Ǵ���100%����ԭ����_______________(�����Dz���������������)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com