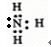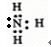X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺
��1������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����
����Ԫ�ط��ű�ʾ����
��2��X��Z�γɵ�3��1�Ļ�����A������Z������������Ӧ��ˮ����B��Ӧ�Ļ�ѧ����ʽ
����ˮ��Һ��
�ԣ������ӷ���ʽ����ԭ��
��
��3����������������Ԫ�أ���Lͬһ���壬������������Ӧ��ˮ���ﻯѧʽΪ
����Ԫ�ع��嵥����H
2��Ӧ����0.5mol��̬�⻯��ʱ������14.87kJ����������д��1mol���������������ϵ��Ȼ�ѧ����ʽ��
��
��4����M������������ʯī��������NaHCO
3��Һ�����Һ���е�⣬����������R��R���ȷֽ����ɻ�����Q��д����������R�ĵ缫��Ӧʽ��
����R����Q�Ļ�ѧ����ʽ��
��