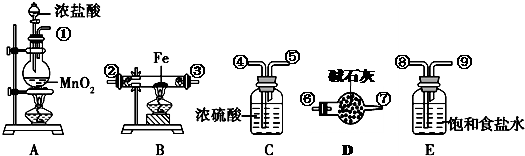
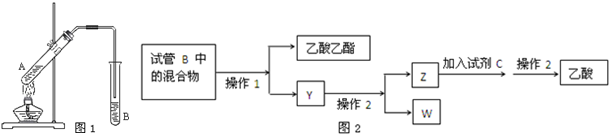
��X��Y��Z����Ԫ�أ���֪��
��X
2-��Y
-����Y����̬�⻯����Ӿ�����ͬ�ĵ�������
��Z��Y����ɻ�����ZY
3��ZY
3��Һ�����ӳ���ɫ��
��ش�
��1����X��ZԪ���γ�ZX
2�������ZX
2�ĵ���ʽ
�����к��еĻ�ѧ����
���ۼ� ���Ӽ�
���ۼ� ���Ӽ�
��
��2����ZY
3��Һ�����ˮ�ɵõ����ɫҺ�壬��Ӧ�����ӷ���ʽ��
����Һ����е�������
abd
abd
����д�����ĸ����
a������ͨ����Һ��ʱ�γɹ����ġ�ͨ·��
b������缫ֱͨ�������һ������Һ����ɫ����
c�����Һ���м�����������Һ����������
d������Һ����ȡ����ɡ����Ⱥ�������������
��3��X�����ڿ�����ȼ������һ����ɫ�д̼�����ζ�����壮
����֪һ�������£�ÿ1mol�����屻O
2��ȫ��������98.0kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ
2SO2��g��+O2��g��=2 SO3��g������H=-196kJ/mol
2SO2��g��+O2��g��=2 SO3��g������H=-196kJ/mol
����2mol��������1molO
2�ڴ������·�����Ӧ���ﵽƽ��ʱ�ų���������176.4kJ����������ת����Ϊ
90%
90%
��
��ԭ��ɫ�д̼�����ζ�������뺬1.5mol Y��һ�ֺ����ᣨ�����ij�γ�����ʵ������ȡ����������Һ��һ�������·�Ӧ��������һ��ǿ���һ�����������1.5��6.02��10
23������ת��ʱ���÷�Ӧ�Ļ�ѧ����ʽ��
SO2+2HClO3=H2SO4+2ClO2
SO2+2HClO3=H2SO4+2ClO2
��
��4��Y������������Ӧˮ����Ļ�ѧʽ��
HClO4
HClO4
��
�ٳ����£���20mL0.1mol?L
-1�ĸ����ʵ�ˮ��Һ��VmL0.1mol?L
-1�İ�ˮ��Ϻ�pH=7����V
��
��
20mL���������������=����
�ڳ����£���pH=2�ĸ����ʵ�ˮ��ҺV
1mL��V
2mL0.01mol?L
-1�İ�ˮ��Ϻ���Һ�����ԣ���V
1��V
2�Ĺ�ϵ����
D
D
A��V
1��V
2 B��V
1��V
2 C��V
1=V
2 D����ȷ��
��ʱ��Һ�д�������Ũ�ȴ�С˳�����Ϊ
c��ClO4-����c��NH4+����c��H+����c��OH-����c��ClO4-����c��H+����c��NH4+����c��OH-�� �� c��ClO4-����c��NH4+��=c��H+����c��OH-��
c��ClO4-����c��NH4+����c��H+����c��OH-����c��ClO4-����c��H+����c��NH4+����c��OH-�� �� c��ClO4-����c��NH4+��=c��H+����c��OH-��
��
��5��Z�ĸ���������Һ�еμ�����HI��Һ�����ӷ���ʽ��
Fe3++3NO3-+12H++10I-=Fe2++5I2+3NO��+6H2O
Fe3++3NO3-+12H++10I-=Fe2++5I2+3NO��+6H2O
��

 �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д� ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д�