
ТЬЗЏЃЈFeSO4?7H2OЃЉЪЧжЮСЦШБЬњадЦЖбЊЕФЬиаЇвЉЁЃФГбЇаЃЕФЛЏбЇаЫШЄаЁзщЕФЭЌбЇЖдТЬЗЏНјааСЫШчЯТЕФЬНОПЃК
FeSO4?7H2OЕФжЦБИ
ИУЛЏбЇаЫШЄаЁзщЕФЭЌбЇдкЪЕбщЪвЭЈЙ§ШчЯТЪЕбщгЩЗЯЬњаМЃЈКЌЩйСПбѕЛЏЭЁЂбѕЛЏЬњЕШдгжЪЃЉжЦБИFeSO4ЁЄ7H2OОЇЬхЃК
ЂйНЋ5%Na2CO3ШмвКМгШыЕНЪЂгавЛЖЈСПЗЯЬњаМЕФЩеБжаЃЌМгШШЪ§ЗжжгЃЌгУЧуЮіЗЈГ§ШЅ
Na2CO3ШмвКЃЌШЛКѓНЋЗЯЬњаМгУЫЎЯДЕг2ЁЋ3БщЁЃ
ЂкЯђЯДЕгЙ§ЕФЗЯЬњаМжаМгШыЙ§СПЕФЯЁСђЫсЃЌПижЦЮТЖШдк50ЁЋ80ЁцжЎМфжСЬњаМКФОЁЃЛ
ЂлГУШШЙ§ТЫЃЌНЋТЫвКзЊШыЕНУмБеШнЦїжаЃЌОВжУЁЂРфШДНсОЇЃЛ
ЂмД§НсОЇЭъБЯКѓЃЌТЫГіОЇЬхЃЌгУЩйСПБљЫЎЯДЕг2ЁЋ3ДЮЃЌдйгУТЫжННЋОЇЬхЮќИЩЃЛ
ЂнНЋжЦЕУЕФFeSO4ЁЄ7H2OОЇЬхЗХдквЛИіаЁЙуПкЦПжаЃЌУмБеБЃДцЁЃ
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЪЕбщВНжшЂйЕФФПЕФЪЧЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЃ
ЃЈ2ЃЉЪЕбщВНжшЂкУїЯдВЛКЯРэЃЌРэгЩЪЧЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЃ
ЃЈ3ЃЉЮЊСЫЯДЕгГ§ШЅОЇЬхБэУцИНзХЕФСђЫсЕШдгжЪЃЌЪЕбщВНжшЂмжагУЩйСПБљЫЎЯДЕгОЇЬхЃЌдвђЪЧЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ ЁЃ
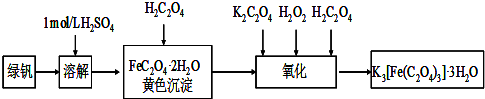
ЃЈЖўЃЉЬНОПТЬЗЏЃЈFeSO4ЁЄ7H2OЃЉШШЗжНтЕФВњЮя
 вбжЊSO3ЕФШлЕуЪЧ16.8ЁуCЃЌЗаЕуЪЧ44.8ЁуCЃЌИУаЁзщЩшМЦШчЯТЭМЫљЪОЕФЪЕбщзАжУЃЈЭМжаМгШШЁЂМаГжвЧЦїЕШОљЪЁТдЃЉЃК
вбжЊSO3ЕФШлЕуЪЧ16.8ЁуCЃЌЗаЕуЪЧ44.8ЁуCЃЌИУаЁзщЩшМЦШчЯТЭМЫљЪОЕФЪЕбщзАжУЃЈЭМжаМгШШЁЂМаГжвЧЦїЕШОљЪЁТдЃЉЃК
ЁОЪЕбщЙ§ГЬЁП
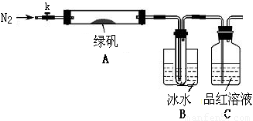
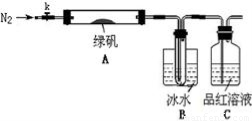
ЂйвЧЦїСЌНгКѓЃЌМьВщзАжУAгыBЦјУмадЃЛ
ЂкШЁвЛЖЈСПТЬЗЏЙЬЬхжУгкAжаЃЌЭЈШыN2вдЧ§ОЁзАжУФкЕФПеЦјЃЌЙиБеkЃЌгУОЦОЋЕЦМгШШгВжЪВЃСЇЙмЃЛ
ЂлЙлВьЕНA жаЙЬЬхж№НЅБфКьзиЩЋЃЌBжаЪдЙмЪеМЏЕНЮоЩЋвКЬхЃЌCжаШмвКЭЪЩЋЃЛ
ЂмД§AжаЗДгІЭъШЋВЂРфШДжСЪвЮТКѓЃЌШЁЩйСПЗДгІКѓЙЬЬхгкЪдЙмжаЃЌМгШыСђЫсШмНтЃЌШЁЩйСПЕЮШыМИЕЮKSCNШмвКЃЌШмвКБфКьЩЋЃЛ
ЂнЭљBзАжУЕФЪдЙмжаЕЮШыМИЕЮBaCl2ШмвКЃЌШмвКБфЛызЧЁЃ
(4ЃЉЪЕбщНсЙћЗжЮі
НсТл1ЃКBжаЪеМЏЕНЕФвКЬхЪЧ?????????????????? ЃЛ
НсТл2ЃКCжаШмвКЭЪЩЋЃЌПЩЭЦжЊВњЮяжага???? ?????????????? ЃЛ
НсТл3ЃКзлКЯЗжЮіЩЯЪіЪЕбщЂлКЭЂмПЩЭЦжЊЙЬЬхВњЮявЛЖЈгаFe2O3ЁЃ
ЁОЪЕбщЗДЫМЁП
ЃЈ5ЃЉЧыжИГіИУаЁзщЩшМЦЕФЪЕбщзАжУЕФУїЯдВЛзуЃК??????????????????????????? ЁЃ
ЃЈ6ЃЉЗжНтКѓЕФЙЬЬхжаПЩФмКЌгаЩйСПFeOЃЌШЁЩЯЪіЪЕбщЂмжабЮЫсШмНтКѓЕФШмвКЩйаэгкЪдЙмжаЃЌбЁгУвЛжжЪдМСМјБ№ЃЌИУЪдМСзюКЯЪЪЕФЪЧ?????????? ЁЃ
aЃЎТШЫЎКЭKSCNШмвК???? bЃЎЫсадKMnO4ШмвК????? cЃЎH2O2???? dЃЎNaOHШмвК
ЃЈЃБЃЉГ§гЭЮлЃЛ
ЃЈ2ЃЉгІИУЬњаМЙ§СПЃЈЛђЗДгІКѓШмвКжаБиаыгаЬњЪЃгрЃЉЃЌЗёдђШмвКжаПЩФмгаFe3+ДцдкЃЛ
ЃЈ3ЃЉгУБљЫЎЯДЕгПЩНЕЕЭЯДЕгЙ§ГЬжаFeSO4ЁЄ7H2OЕФЫ№КФЃЛ
ЃЈ4ЃЉH2SO4ШмвКЁЂSO2ЃЛ
ЃЈ5ЃЉдкCзАжУКѓдіМгвЛЬзЮВЦјДІРэзАжУЃЛ?
ЃЈ6ЃЉbЁЃ
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКЃЈ1ЃЉЬМЫсФЦШмвКгыЗЯЬњаМЕФГЩЗжОљВЛФмЗДгІЃЌЙЪжЛЦ№ШЅгЭЮлЕФзїгУЃЛЃЈ2ЃЉНсКЯЗЯЬњаМЕФГЩЗжКЭКѓБпЕФВНжшПЩжЊЃЌБиаыБЃжЄЬњгаЪЃгрЃЌВХФмБЃжЄШмвКжаУЛгаЬњРызгЃЛЃЈ3ЃЉНЕЕЭЮТЖШЃЌМѕаЁТЬЗЏЕФШмНтЖШЃЛЃЈ4ЃЉСђЫсбЧЬњОЇЬхЪмШШгаЫЎеєЦћЩњГЩЃЌИљОнШ§бѕЛЏСђЕФШлЕуПЩжЊЃЌБљЫЎФмНЋШ§бѕЛЏСђКЭЫЎвКЛЏЃЌдкBжаЗДгІЩњГЩСђЫсЃЛCжаЪЧЩњГЩЕФЖўбѕЛЏСђЪЙЦЗКьШмвКЭЪЩЋЃЛЃЈ5ЃЉЖўбѕЛЏСђвЊНјааЮВЦјДІРэЃЛЃЈ6ЃЉЙЬЬхжагабѕЛЏЬњЃЌШмгкбЮЫсКѓгаШ§МлЬњЩњГЩЃЌвЊЯыМьбщЖўМлЬњЕФДцдкЃЌашРћгУбЧЬњРызгЕФЛЙдадЃЌЪЙЫсадИпУЬЫсМиШмвКЭЪЩЋЃЌЙЪбЁbЁЃ
ПМЕуЃКПМВщбЧЬњРызгКЭЬњРызгЕФМьбщЁЂЖўбѕЛЏСђЕФЦЏАззїгУЁЂЮяжЪЕФжЦБИКЭгІгУЬтФПаХЯЂНтЬтЕФФмСІ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК

| ГСЕэЮя | AlЃЈOHЃЉ3 | FeЃЈOHЃЉ3 | FeЃЈOHЃЉ2 | NiЃЈOHЃЉ2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК

| ГСЕэЮя | AlЃЈOHЃЉ3 | FeЃЈOHЃЉ3 | FeЃЈOHЃЉ2 | NiЃЈOHЃЉ2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| O | 2- 4 |
| O | 2- 7 |
| O | 2- 7 |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com