
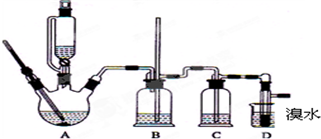
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g?cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -l30 | 9 | -1l6 |
| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
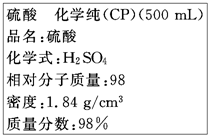 ��ͼ�������Լ�ƿ��ǩ�ϵ����ݣ�
��ͼ�������Լ�ƿ��ǩ�ϵ����ݣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ �� �� Ʒ | �� Һ �� �� | �к��� ��H | |||
| t1 | t2 | ||||
| �� | 50mL 0.55mol?L-1 NaOH | 50mL 0.5mol?L-1HCl | 20�� | 23.3�� | |
| �� | 50mL 0.55mol?L-1 NaOH | 50mL 0.5mol?L-1HCl | 20�� | 23.5�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��K+��SO42-��OH-��Cl- |
| B��Na+��Ag+��Cl-��NO3- |
| C��Ba2+��Na+��OH-��CO32- |
| D��Na+��NH4+��NO3-��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ش��� | X | Y | Z | M | N |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.071 | 0.099 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -1 |
| A��X��Y����������Ӧˮ����ļ��ԣ�Y��X |
| B��M��N�γɵļ����ӵĻ�ԭ�ԣ�N-��M- |
| C����̬�⻯����ȶ��ԣ�HM��H2Z |
| D������������Ӧ��ˮ��������ԣ�H2ZO4��HNO4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com