

��1��FexO��xֵ(��ȷ��0.01)Ϊ____________��
��2�������е�Fen+�ֱ�ΪFe2+��Fe3+,��Fe2+��Fe3+�������У�Fe2+��ռ��������С����ʾ����ȷ��0.001��Ϊ____________________��
��3���˾��廯ѧʽΪ______________��
��4����ij��Fe2+(��Fe3+)��������ҵȾ����O2-Χ�ɵĿռ伸����״��___________��
��5���ھ����У���Ԫ�ص����Ӽ���̾���Ϊ____________m��
����������NaCl����ṹ��1��NaCl��������8��С�����塲����ͼ(1)�����ɵġ�ÿ��С�����庬12��NaCl��ͬ����FexO�����У�ÿ��С�����庬12��FexO�����������С�����ͼ(2)����Fe��O������֣�x=1��

��1��ÿ��С�����������Ϊ��m=��V
=5.71 g��cm-3��(![]() )3
)3
=5.60��10-23 g��
M��FexO��=2m��NA=2��5.60��10-23 g��6.02��1023mol-1=67.4 g��mol-1��
56.0 g��mol-1��x+16.0 g��mol-1��1=67.4 g��mol-1
x=0.92��
��2����1 mol Fe0.92O��N(Fe2+)=y mol,
��N(Fe3+)=(0.92-y)mol�����ݻ������и�Ԫ���������ϼ۴�����Ϊ���ԭ��2��y mol+3��(0.92-y mol)+(-2)��1 mol=0,y=0.76��
��Fe2+��ռ����Ϊ0.76/0.92=0.826��
��3������Fe2+Ϊ0.76,��Fe3+Ϊ0.92-0.76=0.16���ʻ�ѧʽΪ��![]() ��
��
��4��Fe�ھ�������ռ��϶�ļ�����״Ϊ�������塣��ͼ(3)��ʾ��

ͼ(3)
��5����ͼ(1)����Ԫ�����Ӽ�ľ���
r=![]() =1.41��4.28��10-10 m��
=1.41��4.28��10-10 m��![]() =3.02��10-10 m��
=3.02��10-10 m��
�𰸣���1��0.92
��2��0.826��
��3��![]()
��4����������
��5��3.02��10-10


 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ���������ѧУ2011��2012ѧ��߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�022
(1)�۵�е�HF________HI��ԭ��________��
(2)���ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������Ϊ________��
(3)����4�������۵�е��ɸߵ�������Ϊ________(�����)��
�ٽ��ʯ(C��C)����(Ge��Ge)
�۾����(Si��Si)�ܽ��ɰ(Si��C)
(4)������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ����ͼ��ʾ��������ÿ��Na��ͬʱ������________��Cl��������ЩCl�����ɵļ��ι���Ϊ________��������ÿ��Cl����Χ������ӽ��Ҿ�����ȵ�Cl������________����

(5)ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p34s1�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ________��������������Ӧ��ˮ����Ļ�ѧʽ��________��
(6)ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104S2�������ڵ�________���ڣ���________�壬________��Ԫ�أ�Ԫ�ط�����________����ԭ�Ӻ�����________���ܼ��������Ƶ���״��________�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ���������ѧУ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��1�����۵�е�HF HI��ԭ��
��2�������ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������Ϊ
��3��������4�������۵�е��ɸߵ�������Ϊ________ ������ţ�
�ٽ��ʯ��C��C�����ࣨGe��Ge��
�۾���裨Si��Si���ܽ��ɰ��Si��C��
��4��������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ��ͼ��ʾ��
������ÿ��Na+ͬʱ������______��Cl-������Щ Cl-���ɵļ��ι���Ϊ ��������ÿ��Cl-��Χ������ӽ��Ҿ�����ȵ�Cl-����________����
��5��ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s 2s
2s 2p
2p 3s
3s 3p
3p 4s
4s �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ��
��6��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104S2�������ڵ� ���ڣ��� �壬 ��Ԫ�أ�Ԫ�ط����� ����ԭ�Ӻ����� ���ܼ��������Ƶ���״�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��1�����۵�е�HF HI��ԭ��
��2�������ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������Ϊ
��3��������4�������۵�е��ɸߵ�������Ϊ________ ������ţ�
�ٽ��ʯ��C��C�����ࣨGe��Ge��
�۾���裨Si��Si���ܽ��ɰ��Si��C��
��4��������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ��ͼ��ʾ��

������ÿ��Na+ͬʱ������______��Cl-������Щ Cl-���ɵļ��ι���Ϊ ��������ÿ��Cl-��Χ������ӽ��Ҿ����� �ȵ�Cl-����________����
��5��ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s 2s
2s 2p
2p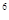 3s
3s 3p
3p 4s
4s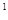 �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��
��6��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104S2�������ڵ� ���ڣ��� �壬 ��Ԫ�أ�Ԫ�ط����� ����ԭ�Ӻ����� ���ܼ��������Ƶ���״�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com