
·ÖĪö £Ø1£©øł¾ŻŹµŃé²Ł×÷µÄ²½ÖčŅŌČ·¶ØČÜŅŗÅäÖĘĖłŠčŅĒĘ÷£»ŅĄ¾Żm=CVM¼ĘĖćŠčŅŖČÜÖŹµÄÖŹĮ棻
£Ø2£©ŅĄ¾ŻŅĘŅŗ”¢Ļ“µÓµÄÕżČ·²Ł×÷½ā“š£»
£Ø3£©·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©ČÜŅŗÅäÖĘ²Ł×÷²½ÖčÓŠ£ŗ³ĘĮ攢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ²Ł×÷£¬Ņ»°ćÓĆĶŠÅĢĢģĘ½³ĘĮ棬ÓĆŅ©³×Č”Ņ©Ę·£¬ŌŚÉÕ±ÖŠČܽā£¬²¢ÓĆ²£Į§°ō½Į°č£¬ĄäČ“ŗó×ŖŅʵ½500mlČŻĮæĘæÖŠ£¬²¢ÓĆ²£Į§°ōŅżĮ÷£¬Ļ“µÓ²¢½«øĆĻ“µÓŅŗŅĘČėČŻĮæĘæÖŠ£¬µ±¼ÓĖ®ÖĮŅŗĆę¾ąĄėæĢ¶ČĻß1”«2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜµĪ¼Ó£¬ĖłŅŌ»¹ŠčŅŖµÄŅĒĘ÷ĪŖ£ŗ500mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£»
Na2CO3•10H2O¾§ĢåÅäÖĘ0.08mol•L-1µÄNa2CO3ČÜŅŗ500mL£¬ŠčŅŖNa2CO3•10H2OµÄÖŹĮæm=0.08mol/L”Į286g/mol”Į0.5L=11.4g£»
¹Ź“š°øĪŖ£ŗ500mLČŻĮæĘ棻½ŗĶ·µĪ¹Ü£»11.4g£»
£Ø2£©ŅĘŅŗŹ±£¬ŅņĪŖČŻĮæĘæĘæ¾±½ĻĻø£¬Ó¦²ÉÓĆ²£Į§°ōŅżĮ÷²Ł×÷£¬¶ØČŻŗó½ųŠŠĻ“µÓ£¬ÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±”¢²£Į§°ō2”«3“Ī£¬²¢½«Ļ“µÓŅŗČ«²æ×ŖŅʵ½ČŻĮæĘæÖŠ£»
¹Ź“š°øĪŖ£ŗŅżĮ÷£»ÉÕ±”¢²£Į§°ō£»
£Ø3£©A£®¶ØČŻŹ±ŃöŹÓČŻĮæĘææĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹA²»Ń”£»
B£®¶ØČŻŹ±ø©ŹÓČŻĮæĘææĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹBŃ”£»
C£®½«ČܽāĪ“¾ĄäČ“µÄČÜŅŗÖ±½Ó×ŖČėČŻĮæĘæŗó¾Ķ½ųŠŠ¶ØČŻ²Ł×÷£¬ĄäČ“ŗóČÜŅŗĢå»żĘ«Š”£¬µ¼ÖĀČÜŅŗÅضČĘ«øߣ¬¹ŹCŃ”£»
D£®¶ØČŻŗ󣬰ŃČŻĮæĘæµ¹ÖĆŅ”ŌČŗó·¢ĻÖŅŗĆęµĶÓŚæĢ¶ČĻߣ¬±ć²¹³ä¼øµĪĖ®ÖĮæĢ¶Č“¦£¬µ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹD²»Ń”£»
E£®³ĘĮæĒ°Na2CO3•10H2O¾§ĢåŅŃŹ§Č„ĮĖ²æ·Ö½į¾§Ė®£¬µ¼ÖĀČÜÖŹŹĒĪļÖŹµÄĮæĘ«“ó£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹEŃ”£»
F£®×ŖŅĘĒ°ČŻĮæĘæĪ“½ųŠŠøÉŌļ£¬ĘæÄŚÓŠÉŁĮæÕōĮóĖ®²ŠĮō£¬¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»ż¶¼²»²śÉśÓ°Ļģ£¬ČÜŅŗÅØ¶Č²»±ä£¬¹ŹF²»Ń”£»
¹ŹŃ”£ŗBCE£®
µćĘĄ ±¾Ģāæ¼²éŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ²Ł×÷¼°Īó²ī·ÖĪöµČ£¬Ć÷Č·ÅäÖĘŌĄķ¼°²Ł×÷²½ÖčŹĒ½āĢā¹Ų¼ü£¬×¢ŅāŅĄ¾Żc=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪöµÄ·½·Ø£¬×¢ŅāČŻĮæŹ¹ÓĆ×¢ŅāŹĀĻī£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
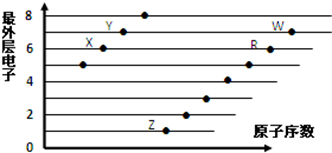
| A£® | XŗĶRŌŚĶ¬Ņ»ÖÜĘŚ | |
| B£® | Ō×Ó°ė¾¶£ŗW£¾R£¾X | |
| C£® | ĘųĢ¬Ēā»ÆĪļµÄĖįŠŌ£ŗX£¾Y | |
| D£® | X”¢ZŠĪ³ÉµÄ»ÆŗĻĪļÖŠŅõŃōĄė×ÓøöŹż±ČĪŖ1£ŗ2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| ×å ÖÜĘŚ | I A | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | O |
| 1 | A | |||||||
| 2 | D | E | G | I | ||||
| 3 | B | C | J | F | H |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
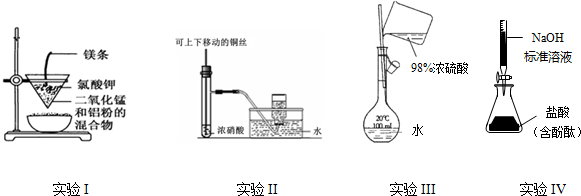
| A£® | ŹµŃéI£ŗÖʱø½šŹōĆĢ | |
| B£® | ŹµŃéII£ŗÖʱø²¢ŹÕ¼ÆNO2 | |
| C£® | ŹµŃéIII£ŗÅäÖĆŅ»¶ØµÄĪļÖŹµÄĮæÅØ¶ČµÄĻ”ĮņĖįČÜŅŗ | |
| D£® | ŹµŃéIV£ŗ²ā¶ØĪ“ÖŖŃĪĖįµÄÅØ¶Č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
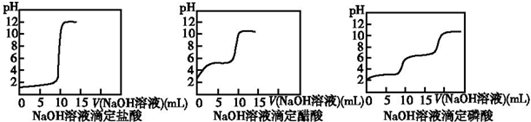
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 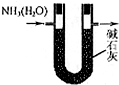 ÓĆČēĶ¼×°ÖĆøÉŌļ°±Ęų | |
| B£® | 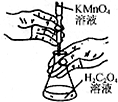 ÓĆČēĶ¼×°ÖĆ½ųŠŠøßĆĢĖį¼ŲČÜŅŗµĪ¶Ø²ŻĖįČÜŅŗŹµŃé | |
| C£® | 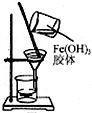 ÓĆČēĶ¼×°ÖĆæÉ·ÖĄėĒāŃõ»ÆĢś½ŗĢåÖŠFe£ØOH£©3ŗĶĖ® | |
| D£® |  ÓĆČēĶ¼×°ÖĆæÉŃéÖ¤ĖįŠŌ£ŗŃĪĖį£¾Ģ¼Ėį£¾±½·Ó |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com