
��10�֣�ʵ�������ռ�����500 mL 0��32mol��L��1��NaOH��Һ��
������� g���ռ���壬����Ӧ���� ��������ƽ���̳�����
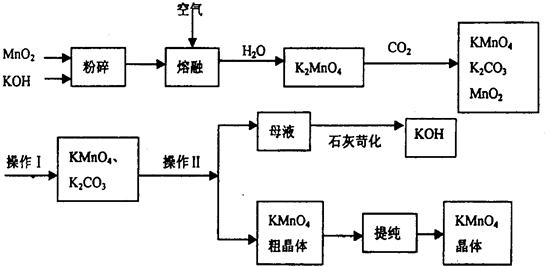
�����ƹ����У�����Ҫʹ�õ������ǣ�����ţ� ��
A���ձ� B����Ͳ C�������� D��l000mL����ƿ E��©��
�۸���ʵ���ʵ����Ҫ�͢����г��������жϣ����ʵ�黹ȱ�ٵ������� �����������ƣ���
�����ڲ����ϵIJ��淶������ʹ������Һ�����ʵ���Ũ��ƫ�͵��� �� ƫ�ߵ��� ��
A������ʱҩƷ������λ�õߵ�
B������ʱ�����
C����Һδ��ȴ��ת������ƿ
D������ƿϴ����δ���
E������ʱ���ӿ̶���
F�����ݺ�תҡ�ȣ�����Һ����ڿ̶��߶�δ��ˮ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �¶ȣ��棩 | O | 10 | 20 | 50 | 60 |
| S��g/lOOgˮ�� | 2.8 | 4.3 | 6.3 | 17.0 | 22.1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
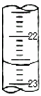
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.50 | 25.40 |
| �ڶ��� | 20.00 | 4.00 | 29.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������һģ ���ͣ��ʴ���
| �¶ȣ��棩 | O | 10 | 20 | 50 | 60 |
| S��g/lOOgˮ�� | 2.8 | 4.3 | 6.3 | 17.0 | 22.1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com